The Urgent Need for a Brain Centered Approach to Geroprotection for Cryonicists

Part I: A Survey of the Problem & a Proposed New Strategy
By Mike Darwin
1964: Dying Then vs. Dying Now
In 1964, the year The Prospect of Immortality1was published, average life expectancy in the United States (US) was 70.2 years.2 Today the average life expectancy has risen to 78.3 years. Americans are living longer than they ever have before.
When cryonics was conceived, the majority of people dying in the US did so with substantially intact brains; the incidence of dementia in people dying at the age of ~70 in the 1960s was ~1%.3 Currently, the incidence of dementia in Americans dying at the average lifespan (78.3) is ~30%. That presents a formidable problem for today’s cryonicists, many of whom are projected to live into their 90s, where the incidence of dementia, primarily from Alzheimer’s Disease (AD) and cerebrovascular disease, rises to 37.4%.4 If you add to that number those cryonicists who will suffer catastrophic brain damaging injuries from stroke, trauma, and neurodegenerative diseases other than AD, the number of cryonics patients who will enter cryopreservation with severely compromised Central Nervous Systems (CNSs) rises to somewhere in the vicinity of ~50%! If you add to that number the ‘losses’ suffered by cryonicists from autopsy and long delays to the start of treatment due to medico-legal constraints, just getting cryopreserved, leaving aside the problems of reanimation, becomes an extremely long-shot proposition.
THE DEAD GERONTOLOGISTS

Figure 1: Some of the dead gerontologists.
Thus, it is pretty apparent that unless medicine makes some truly colossal strides in one hell of hurry, most you now reading this are scheduled for a trip to the freezer – and half of you will make that trip with profound, and likely irreversible damage to your brain. For those of you expecting imminent rescue from Gerontology, I would like to point out that long is the list of names of gerontologists I’ve pinned my hopes on (and in many cases actually known personally), who are now not even footnotes in the contemporary life extension community, but rather, are virtually complete unknowns (Figure1). Nathan Shock,5 Johan Bjorksten, Bernard Strehler,6 Alex Comfort,7 Benjamin Frank, Roy Walford8 and Bill Regelson9 are but a few of the more notable of those names. Most of these men, Alex Comfort10 being an exception, were optimistic about breakthroughs in gerontology occurring in a sufficiently short time frame to save their lives. Unfortunately, they were wrong.
That they were wrong was a tragedy of enormous proportions (and not just for them). But why they were wrong is a matter of profound importance for the medical and economic systems of the entire planet – and especially for us cryonicists, because even with the rapid advances now taking place in the life sciences, medical imaging and computing, the definitive answer to aging is, as we shall see, still almost certainly decades away.
HALFWAY MEDICAL TECHNOLOGY
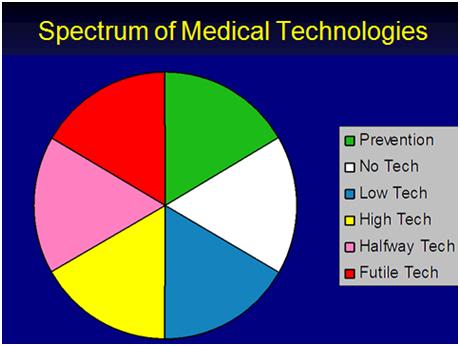 Figure 2: The Spectrum of current medical technologies practiced today.
Figure 2: The Spectrum of current medical technologies practiced today.
The late great physician-philosopher-writer Lewis Thomas first identified the problem in 1974, in his classic book, The Lives of a Cell. Thomas wrote insightfully about four different kinds of medicine we humans are capable of practicing, classifying them as Prevention, No Technology, Low Technology, Halfway Technology and High Technology. I have created a color wheel of Thomas’ medical technologies and added one of my own: Futile Technology – the kind of technology which increasingly characterizes the medicine we practice today (Figure 2).
Prevention, no and low technology medicine are fairly straightforward concepts and do not need our attention here. But High Technology Medicine (HTM), Futile Technology (FT), and especially Halfway Technology (1/2TM), deserve considerably greater scrutiny. For the most part, I will confine in-depth discussion in this article to 1/2TM, however some discussion of HTM and especially FT are merited.
FT is at the root of the impending meltdown of all Western medical and socioeconomic systems. As can be seen in Figure 6, below, there is global and relentless decline in organ function with aging. An inevitable consequence of this is that the aging individual will require more and more supportive medical technology in order (at first) just to remain functional, and eventually in order to simply remain alive. In theory, the demand for (and the cost of) such medical technology is infinite. As will be discussed later, we are currently expending ~16% of the US Gross Domestic product (GDP) on healthcare, and within 5 years, healthcare costs will be in the range of 22% of the GDP! This is simply not sustainable. Even if cancer, heart disease and Alzheimer’s Disease were cured tomorrow, the problem would not only fail to go away, it would be greatly exacerbated, because as long as neuronal attrition continues in aging, even the best maintained extra-cerebral support system will fail due to brain cell loss.
Currently about 1/3rd of every healthcare dollar in the West is spent on people in the last year of their lives. Arguably most of these expenditures are for technological interventions that offer no realistic prospect of therapeutic benefit to the patients to whom they are applied. They not only fail to to restore health, they also fail to either palliate or to restore any meaningful degree of function, and instead only act to prolong the morbid period and its associated suffering. As such, these technologies are futile – they serve no purpose but to preserve unjustified hope at enormous cost, in both dollars and in human suffering. Improvements in slowing, halting or reversing the age-associated degeneration of extra-cerebral tissues in the absence of brain rejuvenation, can thus justifiably be seen as the ultimate in medical futility.
Contemporary ideas of what constitute HTM typically conjure up images of therapies such as artificial hearts, prosthetic limbs, or homologous organ transplantation. In fact, these are halfway technologies which do not definitively cure, and which may properly be viewed as band aids on the underlying pathology: aging. Truly high technology medicine is just beginning to emerge, and consists of technologies such as stem cell therapies, tissue engineering and organogenesis; treatments which will definitively, if not durably repair the patient’s injured soma. However, even HTM has its limits in that however effectively extra-cerebral organs and tissues may be repaired or replaced, these technologies would not seem to offer the promise of conferring rejuvenation, let alone indefinite maintenance of healthy functioning of the brain.
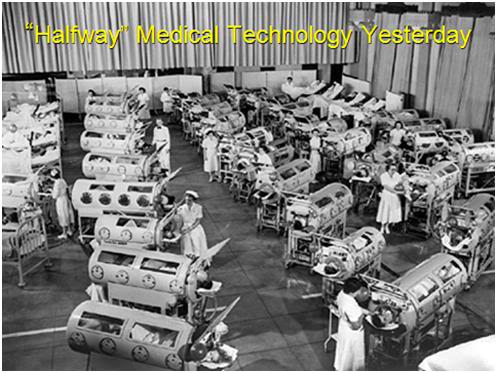
Figure 3: Polio victims on Iron Lung support in a school gymnasium in the mid-1950s.
Thomas elegantly describes Halfway Technology as follows:
“Halfway technology represents the kinds of things that must be done after the fact, in efforts to compensate for the incapacitating effects of certain diseases whose course one is unable to do very much about. By its nature, it is at the same time highly sophisticated and profoundly primitive… It is characteristic of this kind of technology that it costs an enormous amount of money and requires a continuing expansion of hospital facilities… It is when physicians are bogged down by their incomplete technologies, by the innumerable things they are obliged to do in medicine, when they lack a clear understanding of disease mechanisms, that the deficiencies of the health-care system are most conspicuous… The only thing that can move medicine away from this level of technology is new information, and the only imaginable source of this information is research. The real high technology of medicine comes as the result of a genuine understanding of disease mechanisms and when it becomes available, it is relatively inexpensive, relatively simple, and relatively easy to deliver.” —Lewis Thomas11
To understand the difference between 1/2TM and HTM, Thomas used the paradigm of the Polio epidemics of the mid-20th century as an example.12 Today, very few people understand either what the Polio epidemics of the 1950s were like, or the divergent ways that both researchers and clinicians sought to address the scourge. On the one hand, hundreds of thousands of people were contracting polio, with many suffering irreversible bulbar paralysis; which meant that they were unable to breathe. They were conscious and very much alive, but they were unable to use their respiratory muscles to ventilate themselves.
 Figure 4: Jonas Salk, discoverer of the first clinically deployed Polio vaccine.
Figure 4: Jonas Salk, discoverer of the first clinically deployed Polio vaccine.
For many of such paralyzed patients, a relatively new medical device in the form of the Iron Lung represented an opportunity to go on living. In some patients the paralysis retreated, or vigorous physical therapy allowed them to recover sufficiently that they could once again breathe on their own.13 But for many, the Iron Lung was a life sentence of paralyzed immobility inside a cylindrical ‘steel coffin’ as seen in Figure 3.
A minority of scientists at that time believed that it might be possible to defeat Polio by the expedient of a vaccine,14 and so an intense competition for funds began between those who sought to secure more Iron Lungs to support the ever growing legion of patients with respiratory paralysis, and those who sought to understand the fundamental basis of the disease (in the context of their technological era) and treat it by eliminating it.15 In other words, these researchers wanted to get to the root cause of the illness and stop it there, rather than to develop ever more sophisticated Iron Lungs, and other prostheses, to pinch-hit for the muscles rendered useless and atrophied by Polio.
It is, as they say, all history, now. In one of the most rapid translations of bench research to bedside application, Jonas Salk and his colleagues developed a workable Polio vaccine16 which was rolled out for public use in 1955 – the year I was born – just in time to ensure that yours truly would not end up in an Iron Lung, or be ‘lucky’ enough to escape a brush with Polio confined to wheelchair, or using walking braces with a case of ‘simple paralysis,’ as did US President Franklin Delano Roosevelt. 1/2TM is Iron Lungs, and the Salk and later Sabin vaccines, were HTM. Insulin treatment for diabetes, artificial hearts/ left ventricular assist devices, total hip and knee replacements, and drugs for hypertension are also all halfway medicine. They treat the clinical manifestations of disease with varying degrees of efficiency and cost effectiveness, but they do not ever affect a cure.
CONSIDER JANE FONDA
But let’s be clear, 1/2TM is not to be despised, or even ridiculed. Just a few days ago, the movie actress and one-time leftist political activist Jane Fonda, was making the rounds on television to promote her new exercise videos. Fonda is now 74 years old and has a new boyfriend. She also has been successfully treated for breast cancer (with reconstructive surgery) and has had a total hip and knee replacement, as well as replacement of the crystalline lenses of her eyes.17 In Figure 5, at left, we see Fonda she appears today.
 Figure 5: Jane Fonda as she looks today and at right, how she might well look without Halfway Medical Technology.
Figure 5: Jane Fonda as she looks today and at right, how she might well look without Halfway Medical Technology.
If you believe the hair is hers, or that her face has been spared the usual ravages of aging (perhaps from so much Hollywood clean living), well, you’re entitled to your illusions. At right in Figure 5, is what a typical 74 year old looks like in much of the world – including much of the developed Western world where lens implants are not affordable in cataract surgery, joint replacement is not available or affordable, and the bisphosphonates are not an option for preventing osteoporosis. Indeed, Fonda would be lucky to be hobbling about on a cane given her degree of joint degeneration. It is more likely that she would be a customer of the oft advertised ‘Scooter Store’ if it weren’t for her considerable personal wealth and 1/2TM.
Instead, she is doing exercises from her new video on TV, and talking about her sex life, enhanced by that knee replacement (as she commented recently on the Ellen DeGeneres program)18 and that, in no small measure, is because those prosthetics, crude as they are, have allowed her to stay mobile and active. And we can be reasonably sure that another halfway therapy, the bisphosphonate class of drugs, has held osteoporosis in check for her, as well. Without the conditioning made possible by functioning hip and knee joints, and the good eyesight required to use them effectively, these drugs would have only limited benefits. And one more thing, that Ms. Fonda is not sporting a pair of coke bottle eye glasses is the result of the very recent development of implantable replacement lenses for the treatment of cataracts. So, 1/2TM can do some pretty impressive, and some pretty important things, including keeping old people mobile, sighted, cosmetically younger appearing (by decades), and yes, even alive a lot longer, as is the case for Ms. Fonda. All of which is reassuring for those of us rapidly skittering down the rabbit hole into the dark and twisted ‘wonderland’ of old age.
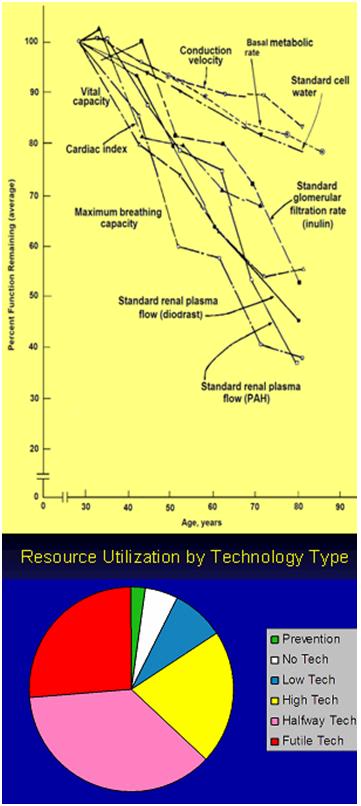 Figure 6: Left, physiological decay as a consequence of aging (data and Graph by Benjamin Shock) and at bottom, the current fraction of medical resource consumption by type of medical technology.
Figure 6: Left, physiological decay as a consequence of aging (data and Graph by Benjamin Shock) and at bottom, the current fraction of medical resource consumption by type of medical technology.
But, we need to be careful, because no technology comes without a price, and as it turns out, 1/2TM carries the ‘Mother of All Price Tags.’ While Jane Fonda looks ~50 on the outside, and singer and actress Cher, at age 64, looks even better, it is critical to understand that this is not the case on the inside. [Here, I must pause to give Cher great credit when she said, just a few weeks ago on an ABC Nightline segment aptly entitled, ‘If I Could Turn Back Time,’3 that “aging sucks” and that “for me, old age gets in the way.”19 ]
The real physiological condition of Jane Fonda (and Cher), as well as that of you or me, can be inferred from the graph in Figure 6, which shows the decay of physiological reserves in multiple organ systems that occur as a result of aging. This data and graph were, by the way, compiled by one of the dead Gerontologists I mentioned at the start of this article, Nathan Shock.20
 Figure 7: Singer-actress Cher at age 64.
Figure 7: Singer-actress Cher at age 64.
Fifty years ago, when I was a boy, a common aphorism was that everyone loses 10,000 brain cells a day – and triple that number for every time they got really drunk (the latter was a very material additional fact in my neighborhood). Since I was ~7 years old at the time, and didn’t drink, I filed this information away, and largely forgot about it. And when I began to seriously study the neurobiology of aging in my 30s, the issue of brain cell loss in aging was something I again considered, but mostly in the context of pathological states – and advanced age – people in their 70s, 80s and 90s had cerebral atrophy; not healthy young men in their 30s. Then there was the reassuring fact that there are ~100 billion neurons in the typical human brain,21,22 and that number seemed comfortably vast, even at a spend-down rate of 10K neurons per day.[1] My erroneous assumption at that time was that brain cell loss must mirror the sharp declines in function seen in other organ systems – most which begin in middle age. It was thus a problem for old people – and I was not old at age 30 (or so I thought).
CEREBRAL ATROPHY IS THE CENTRAL PROBLEM OF AGING FOR CRYONICISTS
It turns out that the old adage about losing 10K of brain cells a day was regrettably much further from the mark than I thought, either when I was 7, or when I was 37. Cerebral atrophy is a big problem in aging, and it turns out the process begins not in middle age, but at approximately 2 years of age – at least for the neurons that comprise the gray matter of the cerebral cortex.23 Brain cell loss and degeneration become morphologically apparent in the brain’s white matter by the time we are in our early 20’s, although there is evidence that more subtle changes have been afoot for much longer.24 Losses in gray matter volume proceed approximately linearly with age in normal aging, and the average gray matter volume decreases from ~390 mL at age 22, to ~300 ml at age 82.25 Total loss in brain mass between age 20 and age 80 is, on average, ~450 g, or roughly 1/3rd of our youthful brain volume. If you are not on the metric system, all you need to know is that an average human brain weighs ~3 pounds when you are age 20, and by the time you are 80, your brain will weigh a pound less. And that is absent disease – if you have Alzheimer’s, hypertension, or atherosclerosis (cardiovascular disease) your losses will be greater – a lot greater.26
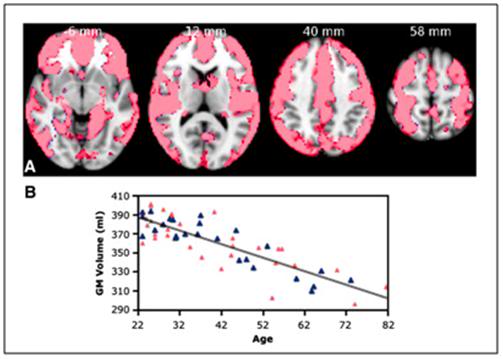
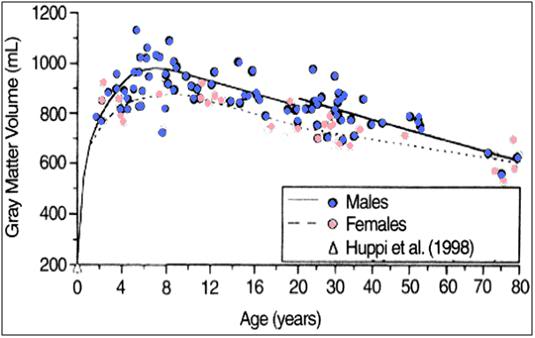
Figure 8: Gray matter loss with aging.
Top: Voxel Based Morphometry (VBM) analysis of gray matter changes in aging. (A) Colored voxels show regions demonstrating significant negative correlations between gray matter volume and age (p < 0.05, fully corrected for multiple comparisons across space). Clusters are overlaid on the MNI152 template brain. Images are shown in radiological convention. (B) Plot to illustrate relationship between age and mean gray matter volume across all significant voxels. The pink triangles represent female subjects. [From: Giorgio, A, Santelli, L, Tomassini, V, Bosnell, R, Smith, S, De Stefano, N, Johansen-Berg, H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943-51.Epub 2010 Mar 6.]
Bottom: Growth and aging changes in gray matter for 116 living healthy individuals. Gray matter volume reached maximum by 6 to 9 years of age and thereafter declined linearly. [From: Courchesne E, Chisum HJ, Townsend J, et al.: Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672.]
 “A good functional analogy might be to consider the brain as an hourglass, with cell loss proceeding at slightly different rates for different individuals, but nevertheless being inexorable, and continuing until the last grain of sand, or in this case neuron, has passed from top to bottom – or that enough have that the integrated functioning of the organism is no longer possible.”
“A good functional analogy might be to consider the brain as an hourglass, with cell loss proceeding at slightly different rates for different individuals, but nevertheless being inexorable, and continuing until the last grain of sand, or in this case neuron, has passed from top to bottom – or that enough have that the integrated functioning of the organism is no longer possible.”
The take-home message in the paragraph above, in case you missed it, is that, “losses in gray matter volume proceed approximately linearly with age in normal aging,” and that’s a real problem, because if you look at the decline in the function of most organ systems in aging in Figure 6, you’ll notice that virtually all of the decay starts at around age 30[2].27 That’s hopeful, in a way, because it suggests that it is a developmental, and therefore likely genetically orchestrated process, and that if we can understand the alterations in gene expression that accompany these declines, we can potentially reverse them. Unfortunately, in the case of brain cells, especially those of the gray matter where we do most of our processing, and where we arguably ‘reside’ as thinking beings, the losses start before puberty, and are quite advanced by the time we are in our in 30s.28-30 The most likely implication of this pattern of life-long and continuous cell loss, is that the brain has no intrinsic capability for robust cellular repair, or replacement. A good functional analogy might be to consider the brain as an hourglass, with cell loss proceeding at slightly different rates for different individuals, but nevertheless being inexorable, and continuing until the last grain of sand, or neuron in this case, has passed from top to bottom – or that enough have that the integrated functioning of the organism is no longer possible.
The near linear loss of gray matter volume and the accompanying heavy losses in gray matter neurons poses a severe problem for the aging cryonicist because they imply that ever more sophisticated advances in 1/2TM, and even HTM, exclusive of true brain rejuvenation, will lead to our becoming neurological struldbrugs,[3] and that is a condition from which not even cryonics can resurrect us.
As is true in much of cutting edge medicine, there is controversy over the clinical significance of these losses in gray and white matter. For instance, it is known that part of procedural learning (how to drive a car, play the piano, or recite your ABCs) is a result of the paring down of connections between some brain cells, and other poorly understood changes in the structure of the white matter. Similarly, selective apoptosis of both gray and white matter neurons seem associated with developmental milestones, including sexual maturation. There has even been dispute over whether or not the ‘normal’ cerebral atrophy of aging is as widespread as previously thought,31 or whether it affects the critical cognitive areas of the brain as badly as it does others. One recent study reported a relative sparing of the hippocampus, the part of the brain critical to memory storage and retrieval. Unfortunately, the dissenters are almost certainly wrong, and cerebral atrophy appears to be global, relentless, and largely pathological.
The controversy, such as it is, stems from the fact that, until very recently with the advent of quantitative brain imaging techniques based on analyses of Magnetic Resonance Imaging (MRI) derived structural data, such as Voxel-Based Morphometry (VBM) and volumetric analyses, it has not been possible to image the regional patterns of grey matter (GM) and white matter (WM) volume loss.32,33
It is surprisingly difficult to get quantitative data of this kind from cadaver brains, and especially difficult to obtain it from the brains of healthy, living subjects when the only unequivocally reliably means of measurement involve removal, fixation and dissection of the subjects’ brains!
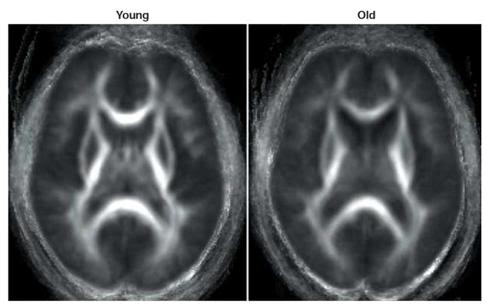 Figure 9: Group-averaged diffusion tensor images of anisotropy of white matter in young and normal elderly. Parallel movement of water molecules through white matter results in anisotropic diffusion, with greater anisotropy (and so greater white matter density) indicated by brighter areas. Older adults tend to show decreased white matter integrity compared with younger adults, with the greatest age-related declines occurring in anterior cortex. (Head, D. et al. Differential vulnerability of anterior white matter in non-demented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb. Cortex (in press). This paper offers a comprehensive DTI study of white matter changes in normal and demented aging and demonstrates the loss of fiber tracts, gliosis and scarring that occur in the so called ‘healthy’ aging brain.
Figure 9: Group-averaged diffusion tensor images of anisotropy of white matter in young and normal elderly. Parallel movement of water molecules through white matter results in anisotropic diffusion, with greater anisotropy (and so greater white matter density) indicated by brighter areas. Older adults tend to show decreased white matter integrity compared with younger adults, with the greatest age-related declines occurring in anterior cortex. (Head, D. et al. Differential vulnerability of anterior white matter in non-demented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb. Cortex (in press). This paper offers a comprehensive DTI study of white matter changes in normal and demented aging and demonstrates the loss of fiber tracts, gliosis and scarring that occur in the so called ‘healthy’ aging brain.
Just as importantly, it has been virtually impossible to image the structural changes in long nerve processes in the brain before the even more recent advent of a technique called diffusion tensor imaging (DTI).34 DTI allows for quantification of alterations in white matter microstructure during aging, and that is something that is otherwise almost impossible to do, even with serial sections of brain tissue examined by light microscopy. Thus, for the first time, literally within the past 2-3 years, we are getting a clearer picture of the neuropathology of ‘normal’ aging, and it isn’t a pretty one.35-40
The development of DTI has been especially useful in documenting age-related changes in white matter, and there is now solid evidence that one of first areas of the brain to undergo age-related white matter decay is the medial temporal lobe (MTL),41 which is the area of the brain that is central to the formation of new memories, and in particular, to the acquisition of new factual information and to remembering events.42-44 Changes in the MTL are first observed (and remain most pronounced in) the perforant path (PP). The PP is so called because it perforates the subiculum[4] and carries input from the entorhinal cortex to the hippocampus, where memory consolidation and encoding are thought to be moderated.45,46
Lesioning of the PP results in defects in memory and learning which are broadly similar to those seen when the hippocampus itself is injured or ablated.47-51 In rats and nonhuman primates there is typically a loss of upwards of 25% of PP axons with aging, and studies of the brains of AD patients, as well as those of patients with mild cognitive impairment (MCI), have revealed widespread synaptic loss, and markedly reduced axon density of PP.53,54
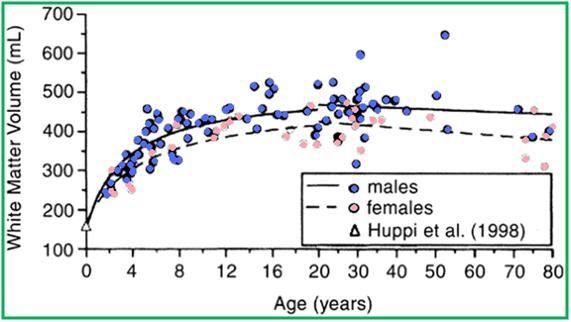 Figure 10: VBM-style analysis of WM changes with age. (A) Colored voxels show regions where WM volume shows a significant linear (blue) or non-linear (green) relationship with age (p < 0.05, fully corrected for multiple comparisons across space). Clusters are overlaid on the MNI152 template brain. Images are shown in radiological convention. (B, C) Plots to illustrate relationship between age and mean WM volume across all voxels showing a significant linear (B) or nonlinear (C) relationship with age. The pink triangles represent female subjects. Giorgio et al. The graph in the green bordered box below shows white matter volume as evaluated by conventional MRI using T1 weighted imaging. This data shows a steady increase in WM volume until age ~40, followed by a modest decline in advanced old age. However, using more sophisticated directional Voxel Based Morphometric imaging, as shown in the purple bordered box at the top of this page, WM changes are revealed to be complex, inhomogeneous between brain hemispheres, and begin in the early 20’s. As can be seen in the VBM white matter graph (purple box) there are, in fact, extensive loses in WM, however they are regional in nature as opposed to the global losses experienced by gray matter as a function of ‘normal’ aging. Growth and aging changes in white matter for 116 living healthy individuals. White matter volume rapidly increased until 12 to 15 years of age, and thereafter increased at a slower rate, plateauing at approximately the fourth decade of life. [From Courchesne E, Chisum HJ, Townsend J, et al.: Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672.]
Figure 10: VBM-style analysis of WM changes with age. (A) Colored voxels show regions where WM volume shows a significant linear (blue) or non-linear (green) relationship with age (p < 0.05, fully corrected for multiple comparisons across space). Clusters are overlaid on the MNI152 template brain. Images are shown in radiological convention. (B, C) Plots to illustrate relationship between age and mean WM volume across all voxels showing a significant linear (B) or nonlinear (C) relationship with age. The pink triangles represent female subjects. Giorgio et al. The graph in the green bordered box below shows white matter volume as evaluated by conventional MRI using T1 weighted imaging. This data shows a steady increase in WM volume until age ~40, followed by a modest decline in advanced old age. However, using more sophisticated directional Voxel Based Morphometric imaging, as shown in the purple bordered box at the top of this page, WM changes are revealed to be complex, inhomogeneous between brain hemispheres, and begin in the early 20’s. As can be seen in the VBM white matter graph (purple box) there are, in fact, extensive loses in WM, however they are regional in nature as opposed to the global losses experienced by gray matter as a function of ‘normal’ aging. Growth and aging changes in white matter for 116 living healthy individuals. White matter volume rapidly increased until 12 to 15 years of age, and thereafter increased at a slower rate, plateauing at approximately the fourth decade of life. [From Courchesne E, Chisum HJ, Townsend J, et al.: Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672.]
Footnotes
[2] The start and the rate of decline are variable, with some organ systems showing deterioration starting in youth. However, for the organ systems shown Figure 6, deterioration does not begin until around age 30.
[3] In Jonathan Swift’s savagely satirical novel Gulliver’s Travels, the name struldbrug is given to those humans in the country of Luggnagg who are born normal, but are in fact immortal. Although the struldbrugs do not die, they do nonetheless continue aging. Swift describes the plight of the struldbrugs in terms almost any resident in an nursing home today (who is still compos mentis) would immediately understand: “when they have completed the term of eighty years, they are looked on as dead in law; their heirs immediately succeed to their estates; only a small pittance is reserved for their support; and the poor ones are maintained at the public charge. After that period, they are held incapable of any employment of trust or profit; they cannot purchase lands, or take leases; neither are they allowed to be witnesses in any cause, either civil or criminal, not even for the decision of meers and bounds.”
[4] The subiculum receives input from CA1 and entorhinal cortical layer III pyramidal neurons and is the main output of the hippocampus. The pyramidal neurons send projections to the nucleus accumbens, septal nuclei, prefrontal cortex, lateral hypothalamus, nucleus reuniens, mammillary nuclei, entorhinal cortex and amygdala and as such, is the principal routing network for information from the hippocampus. The subiculum is also critically involved in the formation of procedural memories.
End of Part 1
References
1) Ettinger, RCW. The Prospect of Immortality, Doubleday, 1964: http://www.cryonics.org/book1.html Retrieved 29 December, 2010.
2) World Development Indicators database: http://www.nationmaster.com/graph/hea_lif_exp_at_bir_tot_yea-life-expectancy-birth-total-years&date=1964#source Retrieved 17 December, 2010.
3) http://aging.senate.gov/crs/aging1.pdf Retrieved 02 February, 2011.
4) Plassman, B, Langa, K, Fisher, G. Wallace, R. Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1–2):125–132. Retrieved 04 February, 2011.
5) Cook, J. Nathan Shock, Pioneer on Aging, New York Times, Obituaries, Published: November 15, 1989: http://query.nytimes.com/gst/fullpage.html?res=950DE1D61E31F936A25752C1A96F948260 ) Retrieved 04 February, 2011.
6) Coles, L. Stephen, “The Life and Contributions of Professor Bernard L. Strehler, Founding Editor-in-Chief of Mechanisms of Aging and Development, Professor of Biology at the University of Southern California [February 21, 1925 May 13, 2001],” Mechanisms of Aging and Development, Vol. 123, pp. 821-5 (2002): Retrieved 03 February, 2011.
7) Rayner, E. Alex Comfort, Obituary, The Guardian, 28 March, 2000: http://www.guardian.co.uk/news/2000/mar/28/guardianobituaries . Retrieved 04 February, 2011.
8) Maugh, II, TH. Obituary: Roy Walford, 79, Obituary; Eccentric UCLA Scientist Touted Food Restriction, Los Angeles Free Press: http://losangelesfreepress.com/obituary-roy-walford-79-eccentric-ucla-scientist-touted-food-restriction/ ). Retrieved 04 February, 2011.
9) Epps, R. Dr. William Regelson, Obituary, Style, Richmond Virginia, 04 February, 2011: http://styleweekly.com/ME2/dirmod.asp?sid=&nm=Articles%2FNews&type=Publishing&mod=Publications%3A%3AArticle&mid=8F3A7027421841978F18BE895F87F791&tier=4&id=A24A05558531439EA6A4A43D345B7CBF . Retrieved 04 February, 2011.
10) Alex Comfort, Wikipedia: http://en.wikipedia.org/wiki/Alex_Comfort . Retrieved 04 February, 2011.
11) Thomas L. The technology of medicine. In: The Lives of a Cell. New York, NY: Viking Press; 1974:31–36.
12) Silver, JK, Wilson, DJ. Polio Voices. Santa Barbara: Praeger Publishers. 2007 p. 141.
13) Wilson DJ. And they shall walk: ideal versus reality in polio rehabilitation in the United States. Asclepio. 2009;61(1):175-92.
14) Smith, JS. Patenting the Sun: Polio and the Salk Vaccine. William Morrow & Co; 1st edition. 1990. ISBN-10: 0688094945.
15) Bookchin, D, Schumacher, J. The Virus and the Vaccine, Macmillan, 2004. ISBN 0312342721.
16) Juskewitch JE, Tapia CJ, Windebank AJ. Lessons from the Salk polio vaccine: methods for and risks of rapid translation. Clin Transl Sci. 2010;3(4):182-5.
17) Fonda, J. My Life So Far, Random House, New York, 2005. ISBN 978-0-375-50710-6 (0-375-50710-8).
18) http://blog.zap2it.com/pop2it/2010/12/jane-fonda-on-breast-cancer-hip-replacement-on-ellen.html Retrieved 23 December, 2010.
19) http://www.hulu.com/watch/194332/abc-nightline-just-cher-if-she-could-turn-back-time Retrieved 04 February, 2011.
20) Shock NW. Age changes in some physiologic processes. Geriatrics. 12;40-48:1957.
21) Pakkenberg, B, Pelvig, D, Marner,L, Bundgaard, MJ, Gundersen, HJG, Nyengaard, JR, Regeur, L. Aging and the human neocortex. Exp. Gerontology. 2003;38:95-99.
22) Pakkenberg, B. Gundersen, HJG. Neocortical neuron number in humans: effect of sex and age. J. Comp. Neurology, 1997;384:312-320.
23) Giorgio, A, Santelli, L, Tomassini, V, Bosnell, R, Smith, S, De Stefano, N, Johansen-Berg, H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943-51.Epub 2010 Mar 6.
24) Hedden T, Gabrieli, JD.. Insights into the aging mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87-96. PMID: 14735112.
25) Courchesne, E, Chisum, HJ, Townsend, J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672.
26) Franke. K, Ziegler, G, Klöppel, S, Gaser, C. Alzheimer’s Disease Neuroimaging Initiative. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage.2010;15;50(3):883-92. Epub 2010 Jan 11. PMID: 20070949.
27) Shock NW. Age changes in some physiologic processes. Geriatrics. 1957;12:40-48.
28) Giorgio, A, Santelli, L, Tomassini, V, Bosnell, R, Smith, S, De Stefano, N, Johansen-Berg, H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943-51. Epub 2010 Mar 6.
29) Franke K, Ziegler, G, Klöppel, S, Gaser C; Alzheimer’s Disease Neuroimaging Initiative. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883-92. Epub 2010 Jan 11. PMID: 20070949.
30) Madden, DJ, Whiting, WL, Huettel, SA, White, E, MacFall, JR, Provenzale, J.M. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage, 2004;21:1174–1181.
31) Burgmans, S, van Boxtel, MP, Vuurman, EF, Smeets, F, Gronenschild, EH, Uylings, HB, Jolles, J. The prevalence of cortical gray matter atrophy may be overestimated in the healthy aging brain. Neuropsychology. 2009;(5):541-50.
32) Basser, PJ, Pierpaoli, CJ. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Magn Reson B. 1996;(3):209-19.
33) Basser, PJ, Mattiello, J, Le Bihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259.
34) Dennis, NA, Cabeza, R. Neuroimaging of healthy cognitive aging. In F. I. M. Craik & T. A. Salthouse (Eds.), Handbook of aging and cognition: Third edition, Psychology Press 2007. ISBN-10: 080585990X
35) Augustinack, JC, Helmer, K, Huber, KE, Kakunoori, S, Zöllei, L, Fischl, B. Direct visualization of the perforant pathway in the human brain with ex vivo diffusion tensor imaging. Front Hum Neurosci. 2010;4:42. PMID: 20577631
36) Yassa, MA, Tugan Muftuler, L, Starka, CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. PNAS. 2010;107:12687–12691.
37) Abe, O., Aoki, S., Hayashi, N., Yamada, H., Kunimatsu, A., Mori, H., et al. Normal aging in the central nervous system: Quantitative MR diffusion-tensor analysis. Neurobiology of Aging. 2002;23(3):433–441.
38) Antonini, A, Leenders, KL. Dopamine D2 receptors in normal human brain: Effect of age measured by positron emission tomography (PET) and [11C]-raclopride. Annals of the New York Academy of Sciences. 1993;695, 81–85.
39) de Leon, M.J, George, AE, Tomanelli, J., Christman, D, Kluger, A, Miller, J. et al. Positron emission tomography studies of normal aging: A replication of PET III and 18-FDG using PET VI and 11-CDG. Neurobiology of Aging. 1987;8(4):319–323.
40) Hedden, T, Riddle, DR, editor. Chapter 11 –in Imaging Cognition in the Aging Human Brain in Brain Aging: Models, Methods, and Mechanisms. CRC Press, 2007.
41) Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010 Nov ;20 (11):1263-90. Review. PubMed PMID: 20928833.) http://dml.ucdavis.edu/articles/ranganath_2010_UnifiedFramework.pdf
42) Wang, WC, Lazzara, MM, Ranganath, C, Knight, RT, Yonelinas, AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68(5):835-42. PMID: 21144998; PubMed.
43) Sauvage, MM, Beer, Z, Ekovich, M, Ho, L, Eichenbaum, H. The caudal medial entorhinal cortex: a selective role in recollection-based recognition memory. J Neurosci. 2010;30(46):15695-9. PMID: 21084625.
44) Bjørnebekk, A, Westlye, LT, Walhovd, KB, Fjell, AM. Everyday memory: self-perception and structural brain correlates in a healthy elderly population. J Int Neuropsychol Soc. 2010;16(6):1115-26. Epub 2010 Oct 15. PMID:20946708.
45) Yassa, MA, Tugan Muftuler, L, Starka, CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. PNAS. 2010;107:12687–12691.
46) Burke, SN, Barnes, CA. Neural plasticity in the aging brain. Nat Rev Neurosci. 2006;7:30–40.
47) Wilson, IA, Gallagher, M, Eichenbaum, H, Tanila, H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670.
48) Milner, B, Squire, LR, Kandel, ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468.
49) Squire, LR, Stark, CEL, Clark, RE. The medial temporal lobe. Annu Rev Neurosci 2004;27:279–306.
50) Witte, MP, Van Hoesen, GW, Amaral, DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci. 1989;9:216–228.
51) Witter, MP, Amaral, DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol. 1991;307:437–459.
52) Witter, MP. The perforant path: Projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res. 2007;163:43–61.
53) Hyman, BT, Van Hoesen, GW, Kromer, LJ, Damasio, AR. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol. 1986;20:472–481.)
54) Scheff, SW, Price, DA, Schmitt, FA, Mufson, EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384.

It was thus a problem for old people – and I was not old at age 30 (or so I thought).
I’m 33, greying hair, and drove to work listening to an internet broadcast discussing neuron loss just this morning. I consider it a present and pressing issue. Glad to see all of this.
The speaker said that men lose many more neurons than women, too. Given that it was my grandmother, not my grandfather, who succombed to senile dementia, I presume that means I am very predisposed to neuron loss by my heredity. Great.
There are many kinds of dementia – Alzheimer’s Disease (AD) is the most common. Risk is heritable, but there are significant environmental interactions as well – concussive head injury being one of them. Three types of AD are directly genetic in origin: APP, PS1, and PS2, all of which are autosomal dominant. Currently the only approved test is for PS1, which is an early onset form of AD that is quite aggressive However all of these mutations only causes about 5% of AD. The presence of the APOE4 gene variant is the greatest currently known genetic risk factor for late-onset “sporadic” AD in a variety of ethnic groups. Caucasian carriers of two APOE4 alleles have 10 to 30 times the risk of developing AD by age 75 as compared to those not carrying any APOE4 alleles. You can find out if you have one, two or no APOE4 alleles with any of a number of Internet-available genetic testing services. Of course, all you will learn is if you are at increased risk of AD, not if you will get it. Interestingly, patients with early onset AD who do NOT have the APOE4 gene, experience much faster progression of disease and die earlier.
As to gender differences in gray matter loss in aging, the answer is not clear. This is a great full text-available paper; Longitudinal Magnetic Resonance Imaging Studies of Older Adults: A Shrinking Brain, by Resnick, et al., which touches on this subject and cites the relevant work in this area to date: http://neuro.cjb.net/content/23/8/3295.full.
Quoting from it:
“Rates of tissue loss were similar in men and women and in older and younger adults. In contrast, the rates of increase in ventricular volume, reflecting central brain atrophy, were significantly greater in older compared with younger individuals. Although we (Resnick et al., 2000) and others (Yue et al., 1997) have observed sex differences in V-CSF in older adults, with older men having larger ventricles than older women, trends toward sex differences in rates of V-CSF enlargement did not reach significance when adjusted for baseline ventricular volume. Although some studies have suggested that men may show earlier increases in ventricular size (Kaye et al., 1992), the magnitude and age at which sex differences appear remain unclear. We have begun enrolling younger BLSA participants into our neuroimaging study to investigate the age at which rates of brain atrophy accelerate in both men and women.”
One thing is clear, and that is that male centenarians and super-centenarians, while less frequent in the population than women, fare considerably better cognitively than do their female counterparts. The reason for this is not known. — Mike Darwin
Interesting. Both of my grandparents made it past 90. My grandfather had very little problem with senility until his last year. So while not a centenarian, I wonder if he benefited from whatever factor helps those other long-living males. If so, I hope I inherited it!
Hello Mike,
Here’s my plan to rejuvenate my ageing brain–and body:
Secure three or four aborted male caucasian first trimester fetuses. Extract all the
stem cells from one, extract only the mesenchymal stem cells from the rest. Slowly
inject all stem cells from one– saving the mesenchymal cells for possible rejection problems.
Here’s how I think this will likely tap into the generational/species immortality: first
and most important, at around birth and shortly after, postmytotic neural stem cells
commit suicide after all the postmytotic neurons are formed. When the fetus is aborted
the developmental program that ‘program’ these cells to commit suicide is aborted!
If these cells are injected into the blood stream of an adult they find there way into the
brain where the ‘adult program’ takes over and they produce YOUNG ADULT TYPE
postmitotic neurons. It looks to me like they will replace our missing neurons and then
replace the old ones as they die off.
Generally, all stem cells and neurons commit suicide when they become damaged so
much by age. The hormone secreting neurons do it at a much faster rate.
The rest of the stem cells will engraft into the proper organs and tissues, produce a dual immune system, produce prime young adult type somatic cells, co-populate with
and replace my old dying stem cells. They should produce ten times or more offspring
than my old cells. Depending on the ratio and output of young and old stem cells the
normal cellular turnover ‘hopefully’ over several years transform this 77 year old into
some kind of a hybrid 20 something…
For more information please google “fetomaternal microchimerism,” also “chimeric
individuals,” and “mesenchymal stem cells and immunity.”
I am actively pursuing doing this experiment on myself and would love hearing your
thoughts. Use the above any way you like but please don’t attribute it to me. If you
like, say from “Gill G Amesh.” ^_^
One of your many admirers,
Roy Yowell
17 Glen Lake Dr
Pacific Grove Ca
93950
831-647-1505