By Mike Darwin
Understanding Hypothermia
The Q10 Rule and Protective Hypothermia
Hypothermia is widely understood to protect against ischemia by virtue of its ability to slow metabolic rate. In man, each 10oC decrement of temperature reduction (below 37oC) results in an approximate halving of metabolic rate, or to be more precise, a reduction of metabolic activity by a factor of ~ 2.2. This consistent reduction in metabolic rate as a function of temperature is known as the “Q10 rule” where Q is oxygen consumption (O2 used per unit of time) which decreases by 1/2.2 with each 10oC drop in body temperature).[2] The Q10 is calculated as:
Where:
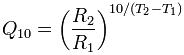
R is the rate
T is the temperature (oC)
(Q10 is a unit-less quantity, as it is the factor by which a rate changes, and is thus a useful way to express the temperature dependence of a process.)
In point of fact, the Q10 rule (or more accurately, the Arrhenius equation from which it was derived) serves as one of the three pillars upon which human cryopreservation rests; [1] i.e., continued reduction in temperature eventually results in the slowing of metabolic and catabolic activity to the extent where, at approximately the boiling point of liquid nitrogen (-196 oC ), all biochemical change is arrested, more or less indefinitely.[3]
The Q10 rule shows surprising constancy across species, with the value being typically between 1 and 3 and, under conditions of hypothermia, has been verified as operational in the brains of rats, dogs and men to ~5oC, at a value of ~2.2.[2] It is important to understand that the decrease in metabolic rate predicted by the Q10 rule is exponential; thus, a decrease in body temperature from 37oC to 17oC results in a decrease in metabolic rate by a factor (1/2.2)2 = 1/4.8. If the Q10 rule is applied to the human brain, using the tolerable limit of cooling before ice formation occurs inflicting freezing damage (~0oC), the predicted slowing of catabolism during ischemia would be such that each hour spent at 0oC would be the equivalent of approximately three and a quarter minutes spent under conditions of normothermic ischemia ([60 min]* 2.2-3.7 = 3.24).
The Q10 rule has important implications for surgery employing deep hypothermic circulatory arrest (DHCA) where there is the need to bound the safe period of cold ischemia with a high degree of confidence. In 1991 Greeley, et al.,[2] derived an equation for approximating the safe circulatory arrest time at any temperature; the Hypothermic Metabolic Index (HMI) which is written as follows:
HMI (min) = (37oC / CRMO2 x XoC)
Where:
CRMO2 = cerebral minute O2 consumption
X = patient cold ischemic temperature
Two important caveats accompany the HMI and they are that the HCT and pH be taken into consideration when making the calculation. HCT determines the hemoglobin decay curve that will take place during the period of hypothermic circulatory arrest (in essence the stored oxygen available in the blood at the time that circulation is interrupted). The pH strategy management strategy management employed during cardiopulmonary bypass (CPB) will affect cerebral blood flow and thus may impact brain metabolic housekeeping. Use of pH stat management[2] results in higher cerebral blood flow (CBF) during CPB and thus, typically, better brain oxygenation and overall metabolic status at the time circulatory arrest begins.[4] Applying the HMI equation to humans yields the spectrum of times vs. temperatures shown in Figure 1 below, and these are in close agreement with what would be predicted on the basis of the Q10 rule. The advantage that the HMI enjoys over the Q10 rule is that it has been empirically “proved” in humans via the Boston Circulatory Arrest Trial.[5],[6]
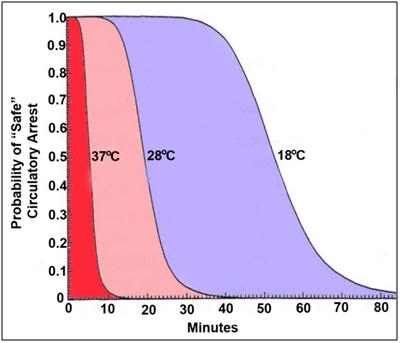
Figure 1: Probable safe circulatory arrest time vs. temperature for humans, as calculated using the Hypothermic Metabolic Index (HMI).
Applying the Q10 rule to cryopatients, or to dogs or rats for that matter, would suggest that 3 hours of cold ischemia is the limit beyond which recovery (absent reparative therapies) would be impossible, and this is indeed the case. Application of the Q10 rule to cryopatients who experience prolonged periods of cold ischemia during Transport, on the order of 24 to 72 hours, would suggest a grim situation pertains; indeed one where decomposition has begun. However, there are problems in extending the Q10 rule over long periods of time at temperatures close to 0oC; with apparent contradictions surfacing in the form of successful preservation of meat and other foodstuffs by simple refrigeration (~4-10oC) for prolonged periods of time,[7] and of even more relevance, the successful storage of human organs (which are comparably sensitive to the brain in terms of cold ischemic injury), for periods of 48 to 72 hours at 1-4 oC[3].[8],[9],[10],[11]
Preservation of ischemia-intolerant organs such as the liver and kidney is made possible not by any sophisticated interruption of metabolism, but by the use of intracellular organ preservation solutions which act primarily by inhibiting cellular edema and scavenging free radicals.[12] So, while the Q10 rule predicts the mammalian brain’s response to ischemia (at least to ~5oC) reasonably well,[13],[14],[15],[16] it does not predict the behavior of other ischemic mammalian organs under the conditions of cold storage for transplantation. Preservation of foodstuffs by refrigeration and prolonged storage of organs near 0oC are possible because the Q10 rule does not take into account several important facts; the first, and probably most important of which, is that much of the metabolic and catabolic activity characteristic of biological systems is facilitated by the catalytic action of enzymes. In fact, biology as we know it is largely an artifact of the greatly accelerated speed of chemical reactions made possible by enzymes, as compared to the rate of reaction predicted on the basis of the Arrhenius equation.[17]
Enzymes are proteins with complex shapes – shapes that are essential to their action as facilitators of chemical reactions – and these shapes are critically dependent upon the structure of the enzymes – in particular, their folding pattern. Profound and ultraprofound hypothermia can destabilize the folding of proteins resulting in a loss of stereospecificity in the case of many enzymes. This phenomenon was first described during cooling of enzymes to below 10oC by Irias and Olmstead in 1969, who referred to it as “cold scission,” or “cold lability,” and noted its effectiveness in halting their biochemical activity.[18] Additionally, phase changes in the non-aqueous lipid components of cells, brought on by deep cooling, can also relieve these molecules of their normal physical mobility and thus their availability for biochemical activity.[19] Additionally, enzymes embedded in lipds that undergo phase change upon cooling to below room temperature may be spatially inhibited by being confined in the solidified membrane.[20]
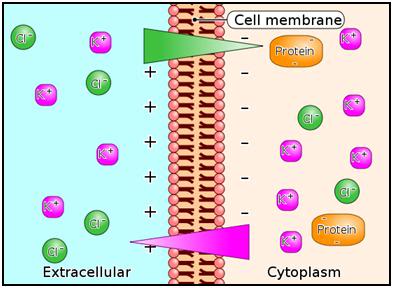 Figure 2: The Gibbs-Donnan Equilibrium; An unstable situation occurs in a solution if one side (I) of the semi–permeable membrane contains a solution consisting of a permeable cation such as K+ with an impermeable anion (Pr–), whereas the other side (II) contains a solution of K+ and Cl–, both of which are permeable to the membrane. The K+ concentrations are equimolar on both sides I and II.
Figure 2: The Gibbs-Donnan Equilibrium; An unstable situation occurs in a solution if one side (I) of the semi–permeable membrane contains a solution consisting of a permeable cation such as K+ with an impermeable anion (Pr–), whereas the other side (II) contains a solution of K+ and Cl–, both of which are permeable to the membrane. The K+ concentrations are equimolar on both sides I and II.
Since side I does not contain Cl–, the Cl– from side II will diffuse along the Cl– concentration gradient from side II to side I. This results in a negative charge on side I relative to side II due to the excess concentration of anions ( Pr– I + Cl–I > Cl–II ) on side I. The Cl– concentrations will not become equal on sides I and II because the negative charge on side I will repel Cl– movement from side II to side I so that side II will have the higher concentration of Cl–. The presence of excess anion in the form of Cl– on side I establishes a negative electrical gradient between side I and side II. The negative electrical gradient attracts K+ to migrate from side II to side I. However, the [K+] on side I now exceeds that of side II , hence K+ will move back from side I to side II along a K+ -concentration gradient.
The net result at equilibrium is that K+ and Cl– move in equimolar amounts together from one side to the other. Each side is electrically neutral within itself due to equimolar concentrations of cations and anions, but there is an unequal concentration of the diffusible ions with [K+] highest on side I and [Cl-] highest on side II.
The effectiveness of intracellular organ preservation solutions provides a clue that, at least near 0oC it may be the case that much of the cold ischemic injury predicted by the Q10 rule (and which is in fact observed to occur) results from not from biochemical activity, per se, but rather from biophysical changes which proceed in the absence of metabolism or catabolism. Under normal metabolic conditions approximately 1/3rd of resting cellular energy expenditures are on ion homeostasis. The protein rich intracellular milieu is positively charged, and the sodium chloride (NaCl- ) rich extracellular milieu is negatively charged (Figure 2). Because NaCl- is osmotically active, movement of NaCl- from the extra- to the intracellular space across the cell membrane (to balance the charge difference represented by the positively charged intracellular protein; the Gibbs-Donan Equilibrium), the result is cellular edema. It is cellular edema, and the biophysics of the Gibbs-Donan Equilibrum, that appear to be a major driver of cold ischemic injury. This is antagonized by intracellular organ preservation solutions by removing most of the offending sodium from the extracellular spaces and replacing it on a roughly equimolar basis with cell membrane impermeable osmotically active species; typically sugars such as lactobionate and raffinose or the sugar-alcohol, mannitol.
Developing a Performance Score for Cryopatient Transport
The Hypothermic Metabolic Index
While thoracic surgeons and neurosurgeons are interested in knowing what the safe limits of DHCA, human cryopreservation professionals have an interest in the reverse problem; how much ischemic injury occurs at a given temperature over a given period of time. This is an important question to answer, even if just approximately, because it offers a quantifiable and reproducible surrogate measure for the quality of care a given patient receives. Absent feedback in the form of neurological deficit or other adverse effects, there is no way for those delivering care to cryopatients to know how well or how poorly they are performing. Such feedback is essential in any enterprise or human undertaking. Absent feedback, quality inevitably deteriorates and science vanishes from the picture.
Measure of Ischemic Exposure (MIX)
By inverting the HMI, or more simply using the Q10 rule in a straightforward calculation of injury vs. ischemic time, it is possible to generate a number for ischemic injury, as opposed to for ischemic protection. In fact, this is exactly what has been proposed by Perry and Harris with their Measure of Ischemic Exposure (MIX)[21] and Equivalent Homeothermic Ischemic Time (E-HIT).[22] Harris’ E-HIT proposal was laid out in an unpublished paper in 2003and is a complex, but potentially very useful mathematical analysis of both cooling in cryopatients, as well as consideration of ischemic injury versus temperature. While neither Harris nor Perry consider the confounding problem of cold inactivation of enzymes in trying to bound ischemic injury, both proposals will serve adequately as a first approximation for surrogate markers (as opposed to reliable objective indicators) of ischemic injury in cryopatients.
Because of its simplicity and ease of use, I have chosen the MIX as the surrogate marker for use here. The mix is a straightforward adaptation of the Q10 rule which normalizes the measurement such that a MIX score of 1 corresponds to 1 hour of cold ischemia at 0oC. Thus, the MIX for 1 hour of ischemia at 10oC would be 2, for 1 hour at 20 oC, 4, at 10 oC, and so on. The MIX for fixed temperatures scales linearly with time so that twice as much ischemia at any given temperature would double the MIX score. This can be expressed by the equation:
MIX = 2T/10t
Where:
t = hours at a constant temperature (oC)
T = patient temperature (oC)
Since patient temperature during cooling is dynamic and continuously changing as a function of time T(t) it is necessary to integrate the expression 2 T/110 over the time period of the ischemic interval; t0 to t0 in which case the MIX iscalculated usingthe following equation:
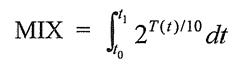
Where:
t = hours at a constant temperature
T = patient temperature (oC)
dt = change in temperature (oC)
The MIX has a simple form for cases involving a constant cooling rate, r degrees per minute starting at the patient’s measured body temperature at the timeof cardiac arrest. For a patient being cooled from 37oC to 0 oC the MIX would be 2.88/r. Thus, for a patient cooled at a rate of 15 oC per hour (T = 2.5 hours to reach 0oC) the MIX would be ~14. For patients who experience peri-arrest cerebral hypoxic or ischemic exposure, this time, beginning with loss of pupillary responsiveness, would be added to the MIX score using the same calculation and using the patient’s body temperature(s) (T) at which the insult was experienced.
The lower the MIX score, the less ischemic injury the patient can be expected to have experienced. Abstracting a performance MIX score from the raw MIX score involves a subjective assessment of what segments of the ischemic insult were avoidable via actions of the human cryopreservation personnel caring for the patient. Unfortunately, this where the opportunity for self delusion, or the delusion of others (unintentional or otherwise), may enter the equation. This is worth exploring, briefly, by using the following scenario.
A terminally ill cryopatient experiences cardiac arrest 45 minutes before Standby personnel arrive on the scene. During that interval he receives no CPS and no refrigeration. The human cryopreservation organization (HCO) may be either blameless, or fully responsible, depending upon a great many factors that were, or were not, under their control. For instance, did the HCO provide proper advice, counseling and support to deal with the possibility of cardiac arrest occurring in their absence (deployment of a field kit, instructions for caregivers on how to perform effective CPS, counseling about the need for ice or other refrigerant to be at hand, etc.)? If not, are they responsible for the extra MIX hit, or some fraction of it? Was their late arrival due to lack of proper medical assessment of the patient that, were it available, would have lead to deployment of the Standby team hours or even days earlier? Or, did the late arrival result from poor logistics by the HCO personnel responsible for booking the flight(s) for the Standby team? If the MIX is to be used as a feedback tool to hold the HCO and the Standby team personnel accountable, then many factors (aside from the fact of the ischemic insult) must be considered, and considered objectively. This is unlikely to happen, absent a set of objective standards for HCO performance, and an outside agency being responsible for evaluating HCO performance with respect to such standards.
Nevertheless, the raw MIX scores for all of a given HCO’s patients for any reasonable given period of time (say on a yearly basis) will necessarily provide a fairly strong indication of the quality of care a member or patient can expect from a given cryonics organization or service provider. For instance, HCOs that provide no Standby, Transport or CPS will have very high MIX scores compared to those that do.
Therapeutic Hypothermia
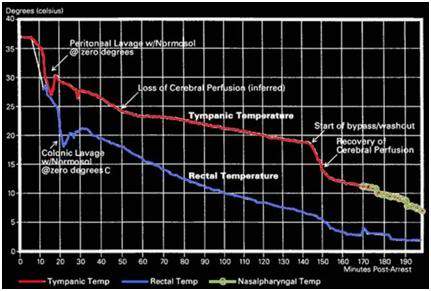 Figure 3: Cooling curve of a cryopatient given excellent cardiopulmonary support (CPS) (and who responded well to that support) who was cooled using external cooling via submersion in a stirred ice water bath, intraperitoneal cooling using ~1-2C o Normosol lavages and, at 145 minutes post arrest, extracorporeal cooling and blood washout.
Figure 3: Cooling curve of a cryopatient given excellent cardiopulmonary support (CPS) (and who responded well to that support) who was cooled using external cooling via submersion in a stirred ice water bath, intraperitoneal cooling using ~1-2C o Normosol lavages and, at 145 minutes post arrest, extracorporeal cooling and blood washout.
Today, yet another factor is confounding the ability of the Q10 rule to delineate the limits of ischemic tolerance (or at least warm ischemic tolerance). That factor is therapeutic, as opposed to preservative, hypothermia. Therapeutic hypothermia may seem to have little relevance to the cryopatient since the primary objective of cooling during Transport is to arrest metabolism and induce a state of preservative hypothermia. However, given current legal and logistic constraints rapid induction of protective hypothermia is not possible for cryopatients. Cooling times to below 20oC are typically in the range of 2-4 hours under good circumstances, and in patients who do not receive intraperitoneal or extracorporeal support may exceed 6 hours, even with AC-DC HI mechanical CPS. The fastest rate at which a cryopatient has been cooled is shown in Figure 3 above, and serves to illustrate that, absent the therapeutic effects of mild and moderate hypothermia, ischemic injury would be far worse in cryopatients. Therefore, a review of the mechanics of therapeutic hypothermia is very much in order.
Over the past 25 years a vast number of therapeutic interventions have shown great promise in animal models of regional and global cerebral ischemia-reperfusion injury (RCIRI & GCIRI) in the laboratory.[23],[24],[25],[26] In the last 6 years alone, over 1000 experimental papers and over 400 clinical articles on pharmacological neuroprotection have been published.[27],[28] However, with one exception, none of these interventions has been successfully applied clinically despite many attempts. [29],[30],[31],[32],[33],[34],[35],[36] The sole exception to this frustrating debacle has been the introduction of mild therapeutic hypothermia (MTH) as the standard of care for a select (and very small) minority of sudden cardiac arrest (SCA) patients.[37],[38],[39],[40],[41],[42],[43]
Since the demonstration by Safar, et al., of the neuro-salvaging effects of mild systemic hypothermia after prolonged cardiac arrest in dogs [44],[45] there has been an explosion of translational research which has lead to a revolution in the understanding and application of mild hypothermia.[46],[47] Once seen only as a protective tool which conferred benefit solely by reducing metabolism, it has become clear that mild hypothermia (33°C–35°C) [48] has therapeutic effects which appear to be primarily anti-inflammatory and anti-apoptotic in nature, and which operate independently of hypothermia’s effect on metabolic rate.[49],[50] Table 1-1 reviews some of the known pro-inflammatory factors inhibited or moderated by mild therapeutic hypothermia (MTH) and documents the supporting literature.
Table 9-1: Inhibition of Injury Cascades by Mild Therapeutic Hypothermia (MTH)
| Reference | Model | Species | T (oC) | Factors |
| Takeda et al (2003) | Global | Gerbil | 31 and 34 | Anoxic depolarization |
| Busto et al (1989b) | Global | Rat | 30 and 33 | Glutamate |
| Dietrich et al (1990) | Global | Rat | 30 and 33 | BBB |
| Kawanishi (2003) | Hemorrhage | Rat | 35 | Edema; BBB; PMNL |
| Kawai et al (2000) | Focal | Rat | 33 | ICAM-1 mRNA; PMNL |
| Wang et al (2002) | Focal | Rat | 30 | ICAM-1; neutrophil and monocyte; microglia |
| Hamann et al (2004) | Focal | Rat | 32 and 34 | MMP-2; MMP-9; m-PA; t-PA |
| Karibe et al (1994a) | Focal | Rat | 33 | Ascorbate; glutathione |
| Kader et al (1994) | Focal | Rat | 33 | NOS; nitrite |
| Toyoda et al (1996) | Focal | Rat | 30 | Neutrophil |
| Chopp et al (1992) | Global | Rat | 30 | HSP-70 |
| Mancuso et al (2000) | Focal | Rat | 33 | HSP-70; C-fos |
| Tohyama et al (1998) | Focal | Rat | 30 | PKC |
| Shimohata et al (2007a) | Focal | Rat | 30 | ePKC |
| Harada et al (2002) | Global | Rat | 32 | CaM kinase II; PKC-a,b,g synaptosome |
| Tsuchiya et al (2002) | Global | Mouse | 33 | Zn2+ |
| Phanithi et al (2000) | Focal | Rat | 33 | Fas; caspase-3 |
| Zhao et al (2007) | Focal | Rat | 33 | Cytochrome c and AIF |
| Karabiyikoglu et al (2003) | Focal
|
Rat
|
33 intra or
post |
iNOS; nNOS
|
| Wagner et al (2003) | Focal | Rat | 33 post | BBB; MMP-9 |
| Inamasu et al (2000) | Focal | Rat | 34.5 post | Neutrophil infiltration; microglia |
| Horstmann et al (2003) | Stroke | Human | 33 post | MMP-9 |
| Horiguchi et al (2003) | Global | Rat | 32 post | Hydroxyl radical |
| Han et al (2003) | Focal | Rat | 33 post | NF-kB; iNOS; TNF-a |
| Van Hemelrijck et al (2005) | Focal | Rat | 34 post | Caspase-3; nNOS |
| Inamasu et al (2000) | Focal | Rat | 34.5 post | Bax |
| Friedman et al (2001) | Global | Rat | 30 intra/post | GluR1A; GluR2B; GluR3C; NMDAR1 |
| Ohta et al (2007) | Focal | Rat | 35 post | Inflammatory genes: osteopontin, early
growth response-1, and macrophage inflammatory protein-3a |
| Luo et al (2007) | Focal | Rat | 33 post | Base-excision repair pathway |
| Preston & Webster (2004) | Global | Rat | 32 post | BBB |
| Liebetrau et al (2004) | Focal | Rat | 32 post | Calpain |
| Hu et al (2008) | Global | Rat | 32 pre/post | of GluR6-PSD95-MLK3 signaling module |
| Deng et al (2003) | Focal | Rat | 33 post | ICAM-1 |
| Karabiyikoglu et al (2003) | Focal | Rat | 33 post | nNOS; iNOS and peroxynitrite |
| AIF, apoptosis-inducing factor; BBB, blood–brain barrier;; HSP-70, heat-shock protein-70; iNOS, inducible nitric oxide synthase; intra, intraischemic hypothermia; MMP-9, matrix metalloprotease-9; M, mouse; NF-kB, nuclear transcription factor kB; NOS, nitric oxide synthesis; nNOS, neuronal nitric oxide synthase; PKC, protein kinase C; PMNL, polymorphonuclear leukocytes; post, postischemic hypothermia; R, rat; S, species; T(1C), intraischemic temperature, unless specified; TNF-a, tumor necrosis factor-a. | ||||
Reproduced with modifications from Zhao, H., Steinberg, GK, Sapolsky, RM., General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cerebr Blood Flow Metab, 2007. 27: p. 1879-1894.
Table 1-1: Intraischemic hypothermia delays or attenuates both ATP depletion (Ibayashi et al, 2000; Sutton et al,1991; Welsh et al, 1990) and anoxic depolarization (Bart et al, 1998; Nakashima and Todd, 1996; Takeda, et al, 2003), it also blocks glutamate release (Busto et al, 1989b; Patel et al, 1994; Winfree et al, 1996), suppresses inflammation (Kawai et al, 2000; Wang et al, 2002), maintains the integrity of the BBB (Dietrich et al, 1990; Huang et al, 1999; Kawanishi, 2003), reduces free radical production (Maier et al, 2002), inhibits protein kinase C translocation (Cardell et al, 1991; Shimohata et al, 2007a, b; Tohyama et al, 1998), inhibits matrix metalloproteinase expression (Hamann et al, 2004), and blocks both necrosis and apoptosis. Intraischemic hypothermia also preserves the base-excision repair pathway, which repairs oxidative damage (Luo et al, 2007). In addition to those cascades directly associated with neuronal injury, hypothermia further blocks astrocyte activity, and inhibits white matter injury. (Colbourne et al, 1997; Dempsey et al, 1987; Kimura et al, 2002). Similarly, postischemic hypothermia blocks free radical generation (Horiguchi et al, 2003), attenuates inflammation (Horstmann et al, 2003; Ohta et al, 2007), prevents BBB permeability (Preston and Webster, 2004), and suppresses caspase activities (Van Hemelrijck et al, 2005). Indeed, a browse through the literature gives an overwhelming impression that hypothermia seems to block every damaging event associated with necrosis or apoptosis. One reason for this impression of pan-inhibition may lie in the causality of ischemic damage. For example, is the inflammatory response the cause of tissue damage or is it induced by brain injury? If it is the latter, then since hypothermia prevents tissue damage, it certainly also prevents the inflammatory response.
The journey from the laboratory to the clinic for MTH has been long and difficult. Seven years after the publication of the prospective randomized trials clearly showing that MTH improves survival and neurological outcome in out-of-hospital cardiac arrest patients, and 6 years after the ILCOR and AHA Guidelines [51] recommended that: “Unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest should be cooled to 32°C to 34°C for 12 to 24 hours when the initial rhythm was ventricular fibrillation (VF),” [41] only a minority of SCA patients are being treated with MTH. In surveys of emergency and critical care physicians conducted in 2005 and 2006, 74% of those responding in the US [52] and 64% of the international respondents indicated they had never used MTH.[53],[54] The use of pre-hospital, in-field MTH, is virtually nonexistent.[55]
No doubt, the commonly cited ‘obstacles’ of lack of institutional protocols, lack of physician education about the benefits and guideline changes, as well as the inevitable inertia that accompanies any paradigm shift in treatment are playing a significant role in the failure of MTH to become the practiced standard of care for the post resuscitation syndrome.[56],[52] However, what is not being said, or considered, is that while MTH as currently practiced represents a large relative improvement in outcome, the benefits are still modest in absolute terms. Only a miniscule subgroup of SCA patients currently can benefit from MTH; and even in its best clinical implementation MTH still fails to rescue ~60% of that sub-group of SCA patients to whom it is applied.[57],[58],[59],[60],[61] This is in stark contrast to what can be achieved with MTH in ameliorating post-ischemic encephalopathy in the laboratory, where post-resuscitation MTH consistently provides rescue with stunning efficacy.[62],[63]
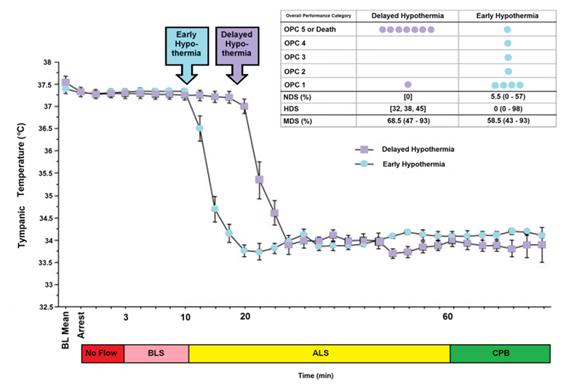 Figure 4: The impact of a delay of 10 min in inducing MHT is a dog model of cardiac arrest followed by 3 min of systemic ischemia, 7 minutes of mechanical CPR and 50 minutes of advanced life support. Hypothermia to 34oC was induced beginning at 10 min post arrest in the early hypothermia group and at 20 min post arrest in the delayed hypothermia group¢. In the early hypothermia group, 5 of 7 surviving dogs were functionally normal (OPC 1 or 2), 1 had OPC 3, and 1 had OPC 4 (coma) at 96 hours of recovery. Histologically, 4 of 8 dogs in this group were normal (HDS 0), 1 had HDS 16, 1 had 22 and 1 had 98. The only surviving dog in the DH group was functionally normal at 96 hours (OPC 1, NDS 0) with an HDS of score of32 (mild injury) Due to early mortality only two other dogs in the delayed hypothermia group were evaluated histologically and their HDS scores 38 and 45, respectively. Dogs in this study were scored by ‘overall performance categories’ (OPC; 1=normal, 2=moderate disability, 3=severe disability but conscious, 4=coma, and 5=death) Neurological function and neurological deficit scores (NDS; 0% to 10%=normal, 100%=brain death). [64],[65] Histological damage scores were obtained by neuropathological examination of 19 discrete brain regions for severity and extent of ischemic neuronal changes, infarcts, and edema. A total brain histological damage score (HDS) >40 represented moderate damage, and HDS >100 represented severe damage.[66] Redrawn from Nozari, A., et al., Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation, 2006. 113(23): p. 2690-6.
Figure 4: The impact of a delay of 10 min in inducing MHT is a dog model of cardiac arrest followed by 3 min of systemic ischemia, 7 minutes of mechanical CPR and 50 minutes of advanced life support. Hypothermia to 34oC was induced beginning at 10 min post arrest in the early hypothermia group and at 20 min post arrest in the delayed hypothermia group¢. In the early hypothermia group, 5 of 7 surviving dogs were functionally normal (OPC 1 or 2), 1 had OPC 3, and 1 had OPC 4 (coma) at 96 hours of recovery. Histologically, 4 of 8 dogs in this group were normal (HDS 0), 1 had HDS 16, 1 had 22 and 1 had 98. The only surviving dog in the DH group was functionally normal at 96 hours (OPC 1, NDS 0) with an HDS of score of32 (mild injury) Due to early mortality only two other dogs in the delayed hypothermia group were evaluated histologically and their HDS scores 38 and 45, respectively. Dogs in this study were scored by ‘overall performance categories’ (OPC; 1=normal, 2=moderate disability, 3=severe disability but conscious, 4=coma, and 5=death) Neurological function and neurological deficit scores (NDS; 0% to 10%=normal, 100%=brain death). [64],[65] Histological damage scores were obtained by neuropathological examination of 19 discrete brain regions for severity and extent of ischemic neuronal changes, infarcts, and edema. A total brain histological damage score (HDS) >40 represented moderate damage, and HDS >100 represented severe damage.[66] Redrawn from Nozari, A., et al., Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation, 2006. 113(23): p. 2690-6.
The primary obstacle to realizing this bonanza in translation research has been the practical impossibility of achieving systemic cooling within the narrow therapeutic window demonstrated in animal models of SCA and resuscitation.[67],[59],[57],[58],[59],[60],[61] If the clinical outcome of MTH was even half that achievable in the laboratory, widespread application would likely have been rapid and uniform; there is rarely resistance to the ‘miraculous’ if it is simple, easy to understand, biophysically well characterized and highly cost-effective. MTH applied immediately post ROSC would be all of these things.[4]
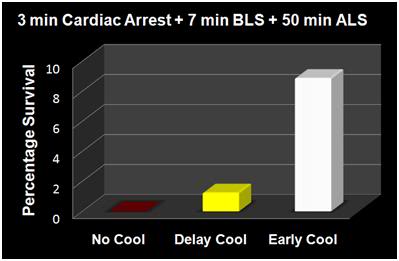 Figure 5: The results of the Nozari, et al., [63] study on the effects of delayed MTH are presented graphically at above, with the addition of historical controls from the literature treated similarly, but with no hypothermia (no survivors). This graphic illustrates the potency of truly rapid post arrest hypothermia increasing survival.
Figure 5: The results of the Nozari, et al., [63] study on the effects of delayed MTH are presented graphically at above, with the addition of historical controls from the literature treated similarly, but with no hypothermia (no survivors). This graphic illustrates the potency of truly rapid post arrest hypothermia increasing survival.
The data in Figures 4 and 5 exemplify what is possible when MHT is induced within its optimum therapeutic window of 0-15 min post ROSC versus a delay of even 10 minutes. In this study by Nozari, et al., of Peter Safar’s group, [63] VF was electrically induced in 17 dogs all of whom were subjected to a period of 3 minutes of no flow beginning when the MAP dropped below 30 mm Hg, followed by 7 minutes of mechanical CPR and 50 minutes of advanced life support during which time VF was maintained and mechanical CPR was continued. Nine animals were treated with rapid (early) induction of MTH to 34oC starting at 10 min post arrest (EH group) (concurrent with the start of ALS to simulate the time course of arrival of EMS paramedics) using a combination of cold IV saline and veno-venous heat exchange. Induction of hypothermia was not begun until 20 min post arrest in the delayed hypothermia group (DH group) which consisted of 8 dogs. Target core temperature was achieved at 6.0±2.7 minutes after the initiation of cooling (3.5 minutes after the start of veno-venous cooling) in both groups. The delay from arrest to reaching ~34oC was 16.6 min in the EH group and 25.4 minutes in the DH group.
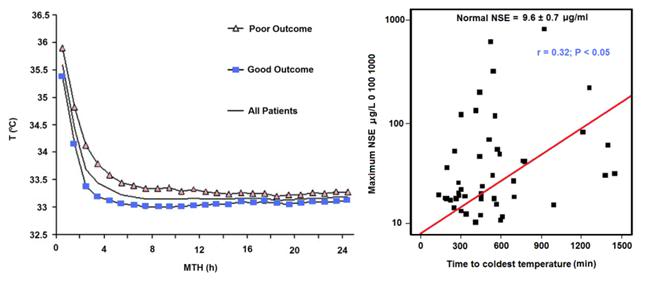 Figure 6: Results of a study of 48 cardiac arrest patients treated with MTH via endovascular cooling. A strong correlation was found between rapidity of cooling and both neurological outcome and serum neuron specific enolase levels. Left: Time course of MTH among patients with good and poor neurological outcome. The curves indicate the course of mean body core temperature during MTH among patients with good (¢) and those with poor (p) outcome as well as in the entire (▬) patient group. Right: Correlation between time to coldest temperature (minutes) and the maximum NSE values (μg/L). Normal serum NSE is 9.6±0.7 μg/L. Redrawn from Wolff B, et al., Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest, Int J Cardiol. (2008).
Figure 6: Results of a study of 48 cardiac arrest patients treated with MTH via endovascular cooling. A strong correlation was found between rapidity of cooling and both neurological outcome and serum neuron specific enolase levels. Left: Time course of MTH among patients with good and poor neurological outcome. The curves indicate the course of mean body core temperature during MTH among patients with good (¢) and those with poor (p) outcome as well as in the entire (▬) patient group. Right: Correlation between time to coldest temperature (minutes) and the maximum NSE values (μg/L). Normal serum NSE is 9.6±0.7 μg/L. Redrawn from Wolff B, et al., Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest, Int J Cardiol. (2008).
After 60 minutes of VF, ROSC was achieved with cardiopulmonary bypass for 4 hours, and intensive care was given for 96 hours. In the early hypothermia group,
7 of 9 dogs survived to 96 hours, 5 with good neurological outcome. By contrast, in the delayed hypothermia group 7 of 8 dogs died of multiple organ failure within 37 hours (P=0.012); 3 animals in secondary VF that was resistant to CPR with antiarrhythmic treatment and repeated defibrillations. Only one dog in the EH group died, and that animal succumbed to single organ failure; pulmonary edema with hemoptysis. This study extends the previous work by this group documenting an optimum therapeutic window for MHT (in dogs) of ~10-15 min.[68],[68],[69] The therapeutic window of MTH after cardiac arrest has been demonstrated to be similarly short in other species.[70],[71],[72],[73]
The dramatic efficacy of MTH in the laboratory made quick converts of the pioneering researcher-clinicians who forged ahead with the application of MTH to SCA in the clinic precisely because it was dramatic; indeed it was as close to the miraculous as interventions in medicine come. The real barrier to translating that ‘miracle’ to everyday practice has been the seemingly intractable problem of achieving cooling over the same time course that has proven so effective in the research setting.
The problem is that the optimum therapeutic window for the treatment of cerebral ischemia-reperfusion injury appears to be in the range of 0 to 15 minutes post ROSC. One of the first follow-up studies on MTH carried out by Safar, et al., demonstrated that in a standardized model of cardiac arrest in dogs a delay in the application MTH of as little as15 min after ROSC abolished most of the benefit.[69],[74] While the work of Bernard, et al., [38] and that of the Hypothermia after Cardiac Arrest Study Group [40] demonstrated that delays in cooling of up to 2-3 hours post ROSC in humans still have sufficient clinical utility to justify the routine application of MTH in a selected group of SCA patients, this benefit is marginal when contrasted to that achievable in the laboratory when MTH is rapidly induced during the first 15 minutes after ROSC.[69] Thus, the optimum clinical benefit of MTH in ischemic and very likely traumatic, CNS injury requires the ability to achieve very rapid core cooling.[75]
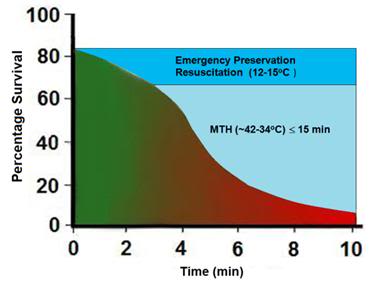 Figure 7: Survival after cardiac arrest declines rapidly as a function of time to ROSC, exhibiting the sigmoidal curve shown at left, above (¢>¢), with essentially all patients failing to survive with normal mentation after arrest intervals of @ 10 min. Application of MTH (¢) within the window of @ 15 min offers the promise of squaring the survival curve in SCA of @ 10 min duration yielding a survival rate of ~65-70% with little or no neurological deficit. Application of deep (10-22oC) or profound hypothermia (5-9oC) (¢) may allow survival after intervals of as long as 1-2 hours of CPR.
Figure 7: Survival after cardiac arrest declines rapidly as a function of time to ROSC, exhibiting the sigmoidal curve shown at left, above (¢>¢), with essentially all patients failing to survive with normal mentation after arrest intervals of @ 10 min. Application of MTH (¢) within the window of @ 15 min offers the promise of squaring the survival curve in SCA of @ 10 min duration yielding a survival rate of ~65-70% with little or no neurological deficit. Application of deep (10-22oC) or profound hypothermia (5-9oC) (¢) may allow survival after intervals of as long as 1-2 hours of CPR.
In the clinical arena the time to reach the target core temperature under ‘good’ circumstances is in the range of 3-4 hrs; not 10 to 30 min, as is the case in the laboratory. Even with such long delays in cooling the adverse effect of delay is still present. Wolf, et al., recently published a study of 49 out of hospital cardiac arrest patients who were treated with MTH (32.0-34.0°C; with a target temperature of 33.0°C) of 24 h duration using endovascular cooling.[61] The study endpoints were neurological outcome on discharge from hospital and serum neuron specific enolase (NSE) levels (a sensitive and specific marker of neuroinjury) at 24 h intervals to 3 days (Figure 6).
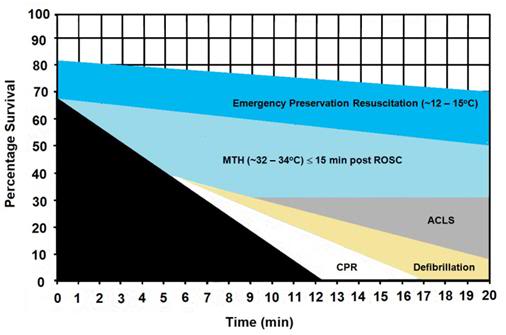 Figure 8: The graph above shows the hypothesized relative effect on survival of effectively administered CPR started at 5 min post-arrest followed by defibrillation at 6 min and ACLS at 8 min post arrest (~30% survival). The light blue shaded area of this graph shows the expected improvement in survival if MTH is induced at the start of ACLS (8 min post-arrest) and target temperature is reached by 15 min post ROSC. The dark blue shaded area shows the potential of Emergency Preservation resuscitation (EPR) using moderate (10-22oC ) or profound (5-9oC) hypothermia to not only square the curve of survival with CPR, but to facilitate survival in patients who would otherwise not benefit from either BCLS or ACLS (i.e., refractory to defibrillation, hypovolemic, etc.). Graphic by M.G. Darwin
Figure 8: The graph above shows the hypothesized relative effect on survival of effectively administered CPR started at 5 min post-arrest followed by defibrillation at 6 min and ACLS at 8 min post arrest (~30% survival). The light blue shaded area of this graph shows the expected improvement in survival if MTH is induced at the start of ACLS (8 min post-arrest) and target temperature is reached by 15 min post ROSC. The dark blue shaded area shows the potential of Emergency Preservation resuscitation (EPR) using moderate (10-22oC ) or profound (5-9oC) hypothermia to not only square the curve of survival with CPR, but to facilitate survival in patients who would otherwise not benefit from either BCLS or ACLS (i.e., refractory to defibrillation, hypovolemic, etc.). Graphic by M.G. Darwin
As is the case in laboratory studies of MTH in cardiac arrest in dogs, Wolff, et al., found that neurological outcomes were binary, with no patients who survived experiencing moderate degrees of disability; patients either recovered well with no or /mild neurological impairment, or experienced severe disability (n = 1) coma or PVS (n = 6). Twenty-eight patients were discharged with a good outcome and a strong correlation was found between good outcome and the time interval from the start of cooling to the lowest temperature (p =.035) and a less robust correlation with the time to reach target temperature (p=.071).
Similarly, NSE levels were found to correlate well with the time required to reach the lowest temperature achieved in each patient (Figure 6).
Even with delays in the start of cooling that averaged 2.5 hrs; and a mean time to reach target core temperature of 6.8 hrs, additional injury accruing from slowness in cooling was still clinically and biologically apparent. Despite the homogeneity of the patients, their arrest times, and their course of treatment, ~60% of the patients in this study either did not survive, or were comatose or PVS.
The Benefits and Limits of ‘Delayed’ MTH: Real World Experience
To understand the benefits and limits of MTH when it is aggressively and competently implemented with currently available technology, it would be hard to find a better example than that of Wake County, SC. Wake County, is located in the northeast central region of North Carolina and is part of the Research Triangle metropolitan area, which consists of Raleigh, Durham, Chapel Hill, and surrounding urban and suburban areas. The area serviced by the Wake County Emergency Medical System (Wake EMS) has a population of 832,970 (as of 2007). The Wake County EMS operates 35 ambulances from 23 locations with 825 ALS personnel; ambulances are staffed with two paramedics 95% of the time and there is always one paramedic responding.[76] In 2002 the Wake EMS answered more than 50,000 medical requests for service. Based on the latest national data Wake County ranks third in the US for recovery from “survivable” cardiac arrests (primarily ventricular fibrillation-ventricular tachycardia). Nationally, the average survival rate is 17% for patients presenting with these arrhythmias.
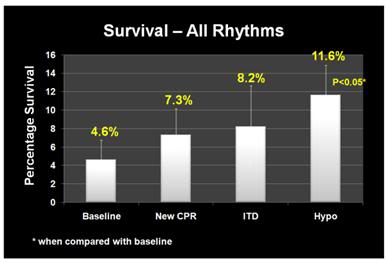 Figure 9: (right): Improvement in overall survival of patients in cardiac arrest in response to the phased introduction of CPR per AHA 2005 Guidelines, use of an impedance threshold device (ITD) and in-field induction of MTH.[77]
Figure 9: (right): Improvement in overall survival of patients in cardiac arrest in response to the phased introduction of CPR per AHA 2005 Guidelines, use of an impedance threshold device (ITD) and in-field induction of MTH.[77]
Beginning in January 2004 Wake EMS initiated a study to evaluate the efficacy of CPR as they then practiced it, and to evaluate the effectiveness of the impending change in the American Heart association (AHA) guidelines for CPR, the introduction of an impedance threshold valve (the ResQPod™) and Wake EMS’ planned implementation of the ILCOR Guidelines for post-arrest MTH.[77],[78] From January 2004 until April of 2005, Wake EMS personnel employed the then extant AHA guidelines, which mandated an emphasis on intubation and a 15:2 compression-to-ventilation ratio; with interruption of chest compressions for ventilation. This period constituted the baseline of the study, and data were collected per protocol; not gathered retrospectively.
During the baseline period survival to discharge from hospital was 2.4% for all patients given CPR and 12.1% for patients with ventricular fibrillation-ventricular tachycardia (VF-VT) arrhythmias. In April 2005 Wake EMS implemented continuous cardiac compression CPR with a 30:2 compression-to-ventilation ratio with emphasis on no, or very minimal, interruption of chest compressions. After 12 months, the overall survival rate had risen to 4%; and had more than doubled to 21.8% for patients who presented in VF-VT.
In April 2006, Wake EMS added the use of an impedance threshold device (ITD) to improve cerebral and coronary perfusion during CPR. Introduction of the ITD resulted in an increase in overall survival to 4.5% and an increase in the survival of patients with VF-VT to 28.5%.
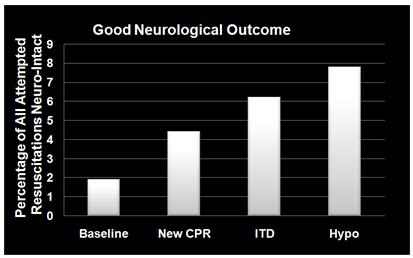 Figure 10: (right): Dramatic improvement in neurologically intact survival as a result of the phased introduction of CPR per AHA 2005 Guidelines, use of an impedance threshold device (ITD) and in-field induction of MTH.[77]
Figure 10: (right): Dramatic improvement in neurologically intact survival as a result of the phased introduction of CPR per AHA 2005 Guidelines, use of an impedance threshold device (ITD) and in-field induction of MTH.[77]
The final phase of the investigative protocol began in October of 2006 when Wake EMS added in-field induction of MTH to the two previous interventions. MTH was induced using a combination of external cooling employing ammonium nitrate-water eutectic ‘instant cold packs’ applied to the axilla and groin, and cold IV saline (1-2oC, 30mL/kg to a maximum of 2 liters) given rapidly via two large bore catheters and/or intraosseous infusion. Criteria for induction of hypothermia were that the patient have ROSC and show no return of consciousness (Glasgow Coma Score (GCS) <8).
Induction of hypothermia was initiated two to three minutes after ROSC. There was heavy emphasis on avoiding over-ventilation and on attempting to maintain end-tidal CO2 (EtCO2) at a minimum of 40 mm Hg. Patients undergoing MHT were sedated with etomidate, paralyzed with vercuronium and given a titrated dopamine drip to maintain mean arterial pressure (MAP) between 90-100 mm Hg. The mean time to target temperature (34oC) in this study was extraordinarily short: 68 minutes (95% CI 47 to 88); compared to the 2-3 hours typically required to induce MTH.
With the combination of continuous compression CPR, use of the ITD and prompt application of MTH, survival rates for the 12 months from October of 2006 to October 2007 had increase to 6.7% overall and 37.4% for patients with VF-VT. The odds of overall survival increased three-fold (95% CI 1.7 to 5.0) and the odds of survival for patients in VF-VT increased 4.3-fold (95% CI 2.2 to 8.6) from the beginning of the study (Figures 9 and 10).The probability of a good neurological outcome increased from 20% at baseline to 80% at the conclusion of the study (Figure 11). In a multivariate analysis, the odds ratios for survival for each phase of implementation were as follows:
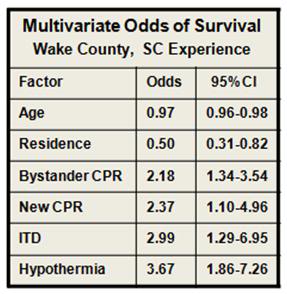 Figure 11: (right): Multivariate odds for all factors in outcome evaluated during the Wake EMS study. MTH was by far the most powerful intervention. As in most previous studies of survival factors associated with CPR age and residence (home versus extended care or assisted living facility) had only modest impact on survival.[77]
Figure 11: (right): Multivariate odds for all factors in outcome evaluated during the Wake EMS study. MTH was by far the most powerful intervention. As in most previous studies of survival factors associated with CPR age and residence (home versus extended care or assisted living facility) had only modest impact on survival.[77]
- New CPR protocol: 2.13 (95% CI 1.12 to 4.04)
- Addition of impedance threshold device: 2.33 (95% CI 1.09 to 5.00)
- Addition of early hypothermia: 3.99 (95% CI 2.19 to 7.27)
- Patients who received bystander CPR 1.79-fold (95% CI 1.18 to 2.72) more likely to survive.
 Figure 12: (right): The Engel-15 portable, (compressor-type) refrigerator/freezer has a 14 L capacity, weighs 11.5 kg and can maintain 12-13 liters of saline at 1-2oC at ambient temperatures as high as 40oC. It retails for ~$380 US. [Photo courtesy of Engel, Ltd., Australia]
Figure 12: (right): The Engel-15 portable, (compressor-type) refrigerator/freezer has a 14 L capacity, weighs 11.5 kg and can maintain 12-13 liters of saline at 1-2oC at ambient temperatures as high as 40oC. It retails for ~$380 US. [Photo courtesy of Engel, Ltd., Australia]
Interestingly, all three elements of the Wake EMS protocol were implemented at a cost of less than $200 per patient. Due to budget constraints, Wake EMS chose simple, inexpensive commercial products for refrigeration of IV fluid and implementation of external cooling, as opposed to more costly products developed specifically for medical application, such as the EMCOOLS surface cooling system (Emergency Medical Cooling Systems, AG, Austria). Saline was kept at the requisite temperature of 1-2oC with a compact, 12V operated, consumer travel refrigerator/freezer (Figure 9-12, Engel-15: http://www.i-m-d.com/) and surface cooling was with generic ammonium nitrate cooling packs.
The Wake County EMS program is extraordinary in every way. It represents the best application of the best available technology by arguably some of the best medical and paramedical personnel in the US. The mean time to target temperature of 68 minutes is unprecedented in any clinical study employing MTH. Of the 359 patients who participated in the study (all comers) after MTH was in place; 25 survived. In the subgroup of 93 patients who presented with VF-VT; 34 survived, with 78% or 27 patients being discharged with a good neurological outcome. Put another way 92% of patients who presented under the most favourable circumstances (VF-VT), treated with the best currently available interventions, at the fastest rate of cooling so far reported, failed to survive or did so with profound neurological debility.
The primary difference between the survivors and the profoundly disabled or dead was the development of the post-resuscitation syndrome and the primary reason for this complication was not comorbidity, or delay in paramedical assistance, but rather delay in the rapidity of cooling which, if achieved within the first 15 min post ROSC, would have offered the prospect of neurologically intact survival in the range of 70-80% in patients presenting with VF-VT, and 30-40% in all comers.
These interventions, remarkable achievements that they are, do not escape from the harsh reality that the 400% increase in survival from cardiac arrest in Wake County, when expressed in absolute terms, means that the number of lives saved increased from ~5 to 25 – out of 395 SCA patients; a huge relative gain, but a comparatively small increase in the absolute number and percentage of lives saved, and minds salvaged. The true life saving potential of MTH remains elusive by virtue of its exceedingly small therapeutic window.
The Problem of Heat Exchange
Because of this minute therapeutic window, there is a pressing need to achieve rapid and durable core cooling of patients during CPR by simple, easily accessible means. External cooling is only effective at reducing core temperatures by 0.15 to 0.25ºC/min in the average patient undergoing CPR (Figure 38) and this is achieved only by complete immersion of patients in a stirred ice water bath.
The most effective external cooling achieved by a commercial, FDA approved system using direct, whole body surface cooling employing circulation of ice water (ThermoSuit,™ Life Recovery Systems, Kinnelon, NJ) is probably the work of Janata, et al., using human human-sized swine.[79]
They were able to achieve core cooling at a rate of 0.3oC/min; however it is important to note that the animals in this study were not in cardiac arrest while undergoing CPR in the presence of profoundly peripherally vasoconstricting agents, such as epinephrine or vasopressin; as would usually be the case during ACLS in humans [51] and which is known to further slow surface cooling.[80]
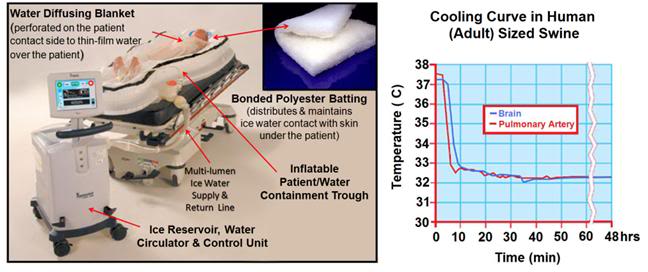 Figure 13: The Life Recovery Systems ThermoSuit™ employs direct ice water contact with the patient’s skin to achieve the maximum possible rate of cooling by external means. The system consists of an inflatable insulating and water containment patient enclosure inside of which the patient rests on a mat of Dacron bonded polyester ‘wool’ which acts to diffuse and film water pumped over the dorsal surface of the patient’s body. Water at 2-4oC is thin-filmed over the ventral surface of the body by a thin, transparent blanket with many hundreds of small perforations through which water under pressure pours out and over the patient. Cold water is recirculated over crushed or cubed ice in an insulated reservoir containing a disposable liner and pumps. Cooling is computer controlled via a thermistor which can be placed in any desired anatomical location. All patient contact items are single-use and disposable (including, as previously mentioned, the pumps) http://www.life-recovery.com/.
Figure 13: The Life Recovery Systems ThermoSuit™ employs direct ice water contact with the patient’s skin to achieve the maximum possible rate of cooling by external means. The system consists of an inflatable insulating and water containment patient enclosure inside of which the patient rests on a mat of Dacron bonded polyester ‘wool’ which acts to diffuse and film water pumped over the dorsal surface of the patient’s body. Water at 2-4oC is thin-filmed over the ventral surface of the body by a thin, transparent blanket with many hundreds of small perforations through which water under pressure pours out and over the patient. Cold water is recirculated over crushed or cubed ice in an insulated reservoir containing a disposable liner and pumps. Cooling is computer controlled via a thermistor which can be placed in any desired anatomical location. All patient contact items are single-use and disposable (including, as previously mentioned, the pumps) http://www.life-recovery.com/.
The obvious problems with this system are its bulk (Figure 13), its high cost ($1,800 per patient for disposables), lack of ease in field deployment (again related to its bulk and weight) and the intrinsic physiological problems associated with the induction of hypothermia via external cooling. As extensively discussed in Section Two, the mammalian body consists of multiple thermal compartments transiently ‘isolated’ from each other by differences in blood flow and heat conductivity.[81] Broadly, these compartments can be classified as strongly and weakly circulated (perfused); corresponding to the body core and periphery. The core tissues receive ~63% of the resting cardiac output (CO) but constitute only ~19% of the total body mass. By contrast, the peripheral tissues receive ~37% of the basal CO and constitute ~81% of the body’s mass (Figure 14).
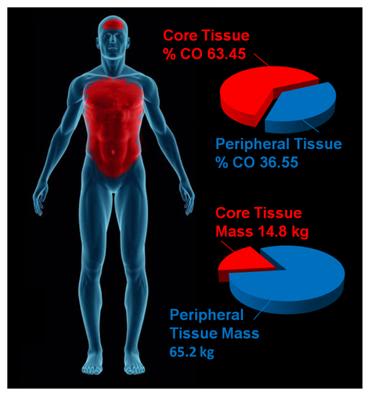 Figure 14: The parenchymatous organs that comprise the visceral core of the body receive an aggregate of ~63% of resting the cardiac output while comprising only ~19% of the body mass. By contrast, the peripheral tissue mass which accounts for ~81% of body mass receive only ~19% of the cardiac output. External cooling profoundly chills peripheral tissues before significantly reducing core temperature. Values for organ and tissue masses were obtained from: IAEA. Compilation of anatomical, physiological and metabolic characteristics for a Reference Asian Man. Volumes 1 and 2. Report IAEA-TECDOC-1005, (Vienna, Austria: International Atomic Energy Agency) (1998), Boecker, BB. References values for Basic Human Anatomical and physiological characteristics for use in radiation protection. Radiation Protection Dosimetry.105(1–4): 571–574;2003, de la Grandmaison GL, Clairand I, Durigon M. Organ weight in 684 adult autopsies: new tables for a Caucasoid population. Forensic Sci Int. 2001 Jun 15;119(2):149-54 and Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr 2007;86:82–91.Values for organ and tissue blood flows were obtained from: Williams, LR, Leggett, RW. Reference values for resting blood flow to organs of man. Clin Physiol Meas. 10:187-212;1989. Graphic by M.G. Darwin
Figure 14: The parenchymatous organs that comprise the visceral core of the body receive an aggregate of ~63% of resting the cardiac output while comprising only ~19% of the body mass. By contrast, the peripheral tissue mass which accounts for ~81% of body mass receive only ~19% of the cardiac output. External cooling profoundly chills peripheral tissues before significantly reducing core temperature. Values for organ and tissue masses were obtained from: IAEA. Compilation of anatomical, physiological and metabolic characteristics for a Reference Asian Man. Volumes 1 and 2. Report IAEA-TECDOC-1005, (Vienna, Austria: International Atomic Energy Agency) (1998), Boecker, BB. References values for Basic Human Anatomical and physiological characteristics for use in radiation protection. Radiation Protection Dosimetry.105(1–4): 571–574;2003, de la Grandmaison GL, Clairand I, Durigon M. Organ weight in 684 adult autopsies: new tables for a Caucasoid population. Forensic Sci Int. 2001 Jun 15;119(2):149-54 and Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr 2007;86:82–91.Values for organ and tissue blood flows were obtained from: Williams, LR, Leggett, RW. Reference values for resting blood flow to organs of man. Clin Physiol Meas. 10:187-212;1989. Graphic by M.G. Darwin
Pathophysiology and Biophysical Limitations of External Cooling
The objective of MTH is to provide protection against ischemia-reperfusion injury to the brain, heart, kidneys and liver; the visceral organs that constitute the strongly circulated core of the body. The peripheral tissues (skin, skeletal muscle, connective tissues and bone) are at once much more resistant to ischemia and less well perfused. External cooling rapidly chills the ischemia-resistant peripheral tissues cooling them profoundly, while failing to provide protection to the vulnerable parenchymatous organs in the body core. This is not only undesirable in terms of its inefficiency; it also poses a number of hazards and risks.[82] Hypothermia is therapeutic in ischemia-reperfusion because it down-regulates the immune-inflammatory response; a response that is vital for host defense, wound healing and hemostasis. Hypothermia, like any major medical intervention that perturbs fundamental physiological processes, carries with it serious risks, as well as benefits. In both animals and humans, hypothermia is markedly immunosuppressive.[83],[84] and interferes with the both the biochemistry of the clotting cascade and the production of platelets and clotting proteins.[85],[86]
In humans perioperative minimal hypothermia (MinH) (36oC) increases the rate of wound infections [87] and prolongs hospitalization. [88] These effects occur in part due to the regional thermoregulatory vasoconstriction MinH induces; which in turn leads to reduced oxygen delivery to injured tissues, [89] inhibition of oxidative killing by neutrophils,[90] and reduced collagen deposition.[88] MinH induces significant suppression of mitogenic responses to Concanavalin A (con A), phytohemagglutinin (PHA), and pokeweed mitogen (PWM) and these changes are known to persist for at least 48 h. The mitogens PHA and ConA activate T cells, whereas PWM stimulates both T and B cells, thus indicating that the suppressive effects of MinH involve a variety of lymphocyte subpopulations. Hypothermia of as little as 1oC significantly inhibits production of interlukins (IL-1б, IL-2, IL-6) and TNF-α in post-surgical patients, [91] and this suppression of cytokine production persists for least 24 hours after even a brief post-operative hypothermic interval.[88] The inhibition of pro-inflammatory cytokine production by IL-1б and TNF-α induce tissue factor which is critical to angiogenesis, collagen elaboration and fibroblast activation; all essential processes in wound repair and hemostasis.[92],[93],[94] Significantly, many of these of adverse effects of post-operative MinH can be prevented by maintaining normothermia in the perioperative period.[88]
In the Hypothermia After Cardiac Arrest Study Group, patients treated with MTH experienced twice the incidence of sepsis. This finding is consistent with other studies where MinH and MTH were found to double the rate of post operative wound infection.[88] A recent meta-analysis of MTH for traumatic brain injury (TBI) found that the incidence of pneumonia was also doubled for patients undergoing MTH.[95]
External cooling causes greater perturbation in hemodynamics than does central cooling, [96] resulting in increased systemic vascular resistance and decreased cardiac index; a phenomenon observed in all of the patients in the Bernard, et al., study that employed MTH for post-arrest cerebral resuscitation.[38] This is particularly undesirable in the setting of MI, CHF and cardiogenic shock. For these reasons, as is the case with any potent therapy, careful attention must be paid to the dose-response curve, and overshoot or excessive regional cooling must be minimized or avoided.[97]
When the tissues of non-hibernating (or unprepared hibernating mammals) are cooled to £20ºC a wide range of deleterious changes occur. The saturated fats which comprise cell membrane lipids undergo phase change, resulting in red and white blood cell rigidity; with accompanying inability to deform and pass through capillaries. Red cell aggregation also occurs and this, in association with reduced flow as a result of vasoconstriction, results in blood sludging and failure of the microcirculation.[98],[99] Profound hypothermia, either local or systemic, results in hemoconcentration as a consequence of translocation of vascular water and electrolyte to the interstitial space.[100],[96],[101],[102],[103] This hemoconcentration further exacerbates regional ischemia in deeply chilled tissues.
Independent of injury from the freezing of water, moderate, profound or ultraprofound hypothermia is known to cause cellular damage which is referred to as ‘chilling injury.’ Chilling injury appears to be a multifactorial process in which alterations of membrane structure (reorganization of lamellar lipid sheets with lateral phase separation between regions of gel phase and regions of liquid crystal phase result in loss of membrane integrity), [104],[105] failure of ion pumping (with consequent disruption of cellular ionic homeostasis), [106],[107] depolymerisation of some elements of the cytoskeleton, [108],[109] generation of free radicals, [110],[111] and metabolic disruption due to selective inactivation of critical enzymes [112] all appear to play a role.
Cooling of tissues to 5ºC for as little as 1 hour has been shown to cause microvascular endothelial damage similar to that observed in ischemia-reperfusion injury; loss of endothelial cell tight junctions, infiltration of capillary and venule walls with leukocytes, and frank extravasation of red cells from injured vessels.[113] A possible reason for the similarity in the histological appearance of chilling injury with ischemia-reperfusion injury may be due to the fact that both types of injury appear to be caused, at least in part, by reactive oxygen species and by disruption of the cytoskeleton.
The molecular changes induced by moderate, profound and ultraprofound hypothermia may also directly compromise endothelial cell integrity. For example, chilling of several types of epithelial cells has been shown to result in disassembly (depolymerisation) of the intracellular microtubules resulting in compromise of the polarized membrane expression and function of some transport proteins in these cells.[114] These functions are slow to return to normal (@20 hr) and are associated with prolonged dysfunction of allografts that have undergone cold preservation storage.[115],[116]
Deep cooling of the peripheral tissues may also result in immunosuppresion in chilled limbs and skin, and possibly impaired hematopoiesis due to localized moderate hypothermia in bone marrow in the cranium, sternum, vertebrae, and to a lesser extent, in the pelvis. [In children, the long bones are the principal repository of hematocytoblasts, and this marrow would also be disproportionately chilled during external cooling.] In contrast to the anti-inflammatory effect of MTH, systemic hypothermia to £28ºC, either accidental or induced has been shown to increase levels of pro-inflammatory cytokines.
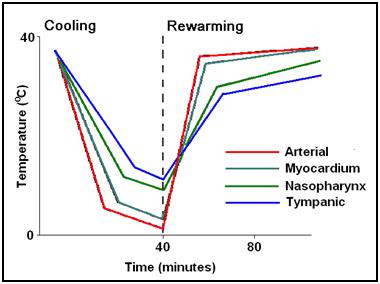 Figure 15: (right): Typical (idealized) cooling and re-warming curve achievable with maximum extracorporeal (cardiopulmonary bypass) cooling.
Figure 15: (right): Typical (idealized) cooling and re-warming curve achievable with maximum extracorporeal (cardiopulmonary bypass) cooling.
Core cooling is thus the gold standard for the induction of systemic hypothermia (mild, moderate, profound or ultraprofound) and external (peripheral) cooling should be used only where there is no other alternative for achieving truly rapid cooling, or maintaining it for the required 24-48 hours following induction.
Consideration of Invasive Core Cooling Methods
 Figure 16: An example of a mobile extracorporeal membrane oxygenation (ECMO) cryopatient support cart. This unit was fabricated by the Alcor Life Extension Foundation and was designed for extended (12-24) hour extracorporeal support of patients in profound or ultraprofound hypothermia.
Figure 16: An example of a mobile extracorporeal membrane oxygenation (ECMO) cryopatient support cart. This unit was fabricated by the Alcor Life Extension Foundation and was designed for extended (12-24) hour extracorporeal support of patients in profound or ultraprofound hypothermia.
Extracorporeal cooling via cardiopulmonary bypass (CPB) [117] or high flow veno-venous heat exchange [118],[119] allows for cooling rates of 0.8ºC/min to 1.0ºC/min (Figure 15) However, it cannot be applied rapidly enough given existing logistic and regulatory constraints. CPB requires complex hardware and highly skilled personnel who must maintain their clinical reflexes by practicing perfusion on a regular (preferably daily or at least weekly) basis. Even in centers of excellence, with a highly skilled, rapid-response CPB team at the ready (including a well-practiced surgeon or cardiologist), the soonest CPB can be initiated after cardiac arrest is typically 15-20 minutes.[120],[121]
The use of emergency CPB applied in this time frame is largely confined to patients undergoing cardiac catheterization and/or revascularization (i.e., angioplasty or stent placement) who arrest in the cardiac catheterization lab. When such patients experience cardiac arrest that is refractory to treatment with drugs and defibrillation – they are usually otherwise healthy – they have normally functioning lungs, normal fluid balance and fluid distribution (are neither dehydrated nor edematous from fluid overload), and have failure of a only a single organ – the heart. Even under such ‘ideal’ conditions, CPR is often inadequate to maintain brain viability during the brief interval between cardiac arrest and the start of CPB.
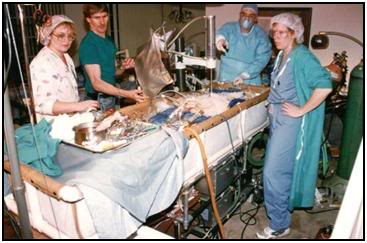 Figure 17: Cryopatient undergoing ECMO and blood washout in the home. Even under ideal circumstances cardiopulmonary bypass takes in excess of 30 minutes to initiate
Figure 17: Cryopatient undergoing ECMO and blood washout in the home. Even under ideal circumstances cardiopulmonary bypass takes in excess of 30 minutes to initiate
While veno-venous extracorporeal cooling is less technically demanding, it still requires skill-intensive vascular access under field conditions, and reported rates of cooling are modest; ~0.012ºC/min. [122],[79] Both of these techniques may require anticoagulation and certainly require coagulation monitoring; which again is a barrier to in-field application.
Administration of large volumes (40mL/kg) of chilled intravenous fluid has also been used to reduce core temperature in the field in patients undergoing CPR. However this method is extremely slow (~0.058ºC/min),[123],[124],[125] and is sharply constrained by the maximum volume of fluid that can be administered.
The effectiveness of intravascular fluid administration in achieving durable core cooling is also a function of body composition. Obesity, which has become epidemic in the developed world, is now rapidly becoming a global problem with 1.1 billion adults worldwide classified as overweight, and 312 million of them as obese by the World Health Organization (WHO).[126] Vascular volume does not increase proportionally to increase in body weight; in fact, the vascular volume to body weight ratio falls toward an asymptotic value of approximately 45 ml/kg in the obese human.[127] Thus, the use of cold intravenous infusions will necessarily be even less effective in the obese than might be expected since the maximum volume of fluid that may be safely given does not increase linearly with body weight. Fat is also an extraordinarily good insulator, and obesity often unfavorably alters body surface to volume area. Both of these factors combine to dramatically reduce the speed and overall effectiveness of external cooling.
The limits of efficiency of core cooling that can be achieved by irrigating the peritoneal or pleural spaces with cold liquid are in the range of 0.05 to 0.3ºC/min [128],[129] and both of these techniques are invasive, require considerable technical skill, and carry with them risks of serious iatrogenic consequences.
As noted earlier, the ideal way to address these needs would be extracorporeal membrane oxygenation (ECMO) and cooling. However, the practicality of rapidly bringing this demanding technology to bear in the EMS setting is negligible. Surgery (or time-consuming percutaneous vascular access; at or beyond the optimum therapeutic window of 15 min post ROSC) is required to access the circulation and this cannot be accomplished under field conditions. Experience with emergent initiation of ECMO in patients presenting for experimental cryopreservation (Figure 38) are probably representative of the time required to achieve extracorporeal support under field conditions for patients with SCA. Such patients typically experience a delay of 60 to 80 minutes (and not infrequently longer) after the initiation of closed chest CPR before the application of CPB, even under optimum circumstances.[130],[131],[132]
Thus, what is needed for cryopatients, patients who have suffered prolonged (³5 min) cardiac arrest, or in whom ROSC cannot be effectively established, is the ability to rapidly induce hypothermia via core cooling simply and noninvasively. In addition to moderating the injury cascade initiated by ischemia-reperfusion, mild (33-35oC) or moderate (28-32oC) hypothermia can indirectly act to improve gas exchange and hemodynamics by bringing the patient’s cerebral and systemic metabolic demands closer to those that can be delivered by CPR.
The clinical experience of the Wake EMS and in-house research into accelerated non-invasive cooling methods will be combined in the next section to allow far greater rates of cooling of cryopatients than have been possible in the past.
Induction of Hypothermia in the Cryopatient
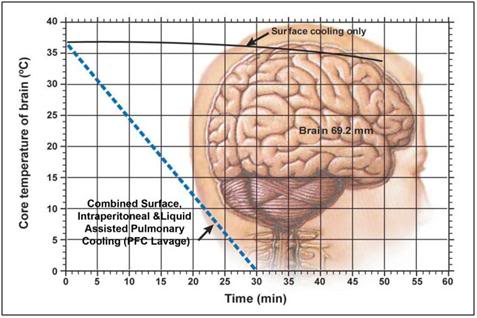 Figure 18: Idealized optimal core brain cooling curve. The solid black line (___) indicates the brain core (69.2 mm depth) cooling curve achieved using external cooling with ice water immersion during CPS. The broken blue line (—-) shows the theoretical cooling curve that might be possible by combining surface cooling with either cardiopulmonary bypass (CPB) cooling, or with liquid assisted pulmonary cooling (LAPC) and intraperitoneal cooling using ~1-2oC physiologic salt solution, such as Normosol-R.™
Figure 18: Idealized optimal core brain cooling curve. The solid black line (___) indicates the brain core (69.2 mm depth) cooling curve achieved using external cooling with ice water immersion during CPS. The broken blue line (—-) shows the theoretical cooling curve that might be possible by combining surface cooling with either cardiopulmonary bypass (CPB) cooling, or with liquid assisted pulmonary cooling (LAPC) and intraperitoneal cooling using ~1-2oC physiologic salt solution, such as Normosol-R.™
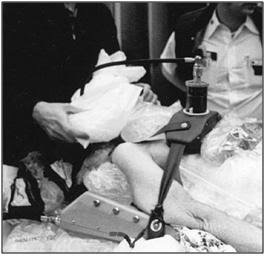 Figure 19: Maintaining contact between the patient and the refrigerating ice bags was virtually impossible in the early days of human cryopreservation since the bags continually slide off the patient during Transport.
Figure 19: Maintaining contact between the patient and the refrigerating ice bags was virtually impossible in the early days of human cryopreservation since the bags continually slide off the patient during Transport.
In the early days of cryonics, post-cardiac arrest cooling was exclusively “external” cooling which was achieved by covering the patient with plastic bags containing crushed or small cubed ice (Figure 19).[133], [134],[135] This approach had the obvious advantage of being simple, straightforward to implement, and very inexpensive. Unfortunately, years of actual field experience with this approach have disclosed a number of serious limitations and problems:
1) Heat exchange is greatly attenuated by both the insulating properties of the plastic and the reduction of convective transfer due to containment of the ice water generated from the melting ice.
2) It is difficult to properly and completely pack the patient in ice because the bags are constantly falling off or not staying properly positioned, i.e. in good contact with the patient’s skin. Maintaining ice bags around the head and neck is a particular problem.
3) It is virtually impossible to get ice packs under the patient, which means that 35% to 45% of the patient’s surface area is unavailable for heat exchange.
4) Ice bags leak and sweat which cause water to wet the patient and drip off the cot or gurney during transport. This presents both an immediate safety hazard (creating slippery floors and a potential electrical hazard) and also serves to contaminate staff and the working environment with potentially infectious fluids.
The PIB was developed after in-field core temperature measurements during the transport of a human cryopreservation patient employing ice bags to facilitate external cooling disclosed that this method was grossly unsatisfactory resulting in a core cooling rate of approximately 0.05°C/min during the first 4 hours of cooling.[134], [136], [137], [138] Subsequent cases confirmed very slow rates of cooling using externally applied plastic bags filled with water ice ranging between a high of 0.12°C/min to a more typical 0.064°C/min.[134] For these reasons, a lightweight, Portable Ice Bath (PIB) was developed by the author to allow for direct contact of ice water with essentially the entire surface area of the patient to simulate rates of heat exchange presumably encountered in cold water drowning where recovery of adults without neurological deficit after 20-40 minutes or more of submersion in ice water has been repeatedly clinically documented.[139],[140], [141],[142] The rate of heat-loss for an adult human in water is 32 times that of in air5. Indeed, in such cases of cold water drowning, it is sometimes possible to successfully resuscitate adults who have been chilled in the absence of any cardiopulmonary support for up to 30 minutes.[143],[144],[145] Children have been known to recover following up to 66 minutes of cold water drowning.[146]
Determining the rate of cooling likely to be achieved with the PIB, particularly with the addition of convection cooling by stirring or circulating the PIB water around the patient was important prior to expending the considerable resources required and logistic difficulties to be overcome if these techniques were to be implemented routinely during Transports. A careful examination of the literature was undertaken by the author circa 1987 to determine if there were any published data on the rate(s) of core cooling likely to be feasible with these modalities.
Unfortunately, the only published data were those of the SS (Schutzstaffel) Nazi physicians Holzloehner, Finke, and Rascher, et al., conducted in 1942 on prisoners in the German concentration camp Dachau as part of an effort to understand the mechanisms and time course of hypothermia (as well as methods of safe re-warming) in Luftwaffe pilots downed in cold North Sea water (Figure 20). Most of the actual work was conducted by Sigmund Rascher, M.D. and was summarized after its post-war recovery by Major Leo Alexander of the U.S Army Medical Corps in 1946.[147] This document, now referred to as the “Alexander Report,” contains detailed information in both graphic and tabular form on the effects of convective cold water external cooling on human prisoners.
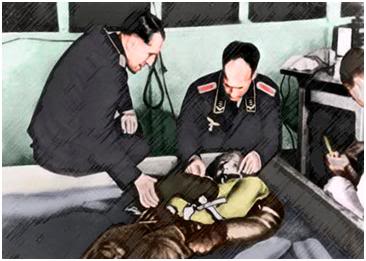 Figure 20: Sturmbannfuerher Dr. Sigmund Rascher (R) and Dr. Ernst Holzhoen (L) experimenting on a prisoner from Dachau using cold-water immersion to induce hypothermia followed by attempted re-warming after loss of consciousness. (Art from photo via Vad Yashem, Jerusalem, Israel.)
Figure 20: Sturmbannfuerher Dr. Sigmund Rascher (R) and Dr. Ernst Holzhoen (L) experimenting on a prisoner from Dachau using cold-water immersion to induce hypothermia followed by attempted re-warming after loss of consciousness. (Art from photo via Vad Yashem, Jerusalem, Israel.)
At this time, the citation and use of this data are extremely controversial and their relevance, integrity, and scientific usefulness have been called into question.[148] There is no question in the author’s mind that the work of Holzloehner, et al., constitutes an atrocity and a crime against humanity of the worst kind. One of the principal criticisms of the scientific validity of this work has been the observation that many of the more than 300 victims of this research were chachectic and weakened from malnutrition and frank starvation making their physiological responses non-representative of that of the healthy German aviator. Ironically, it is just this criticism that makes the data obtained in this study uniquely valuable to human cryopreservation.
Data from the Alexander Report must be interpreted with caution and within the context of both the typical conditions under which human cryopreservation takes place and more recent data on the effects of cold water immersion on human subjects which is unquestionably both ethically and scientifically sound.[149],[150],[151]
Several important caveats apply: Cooling curve data obtained by Holzloehner, et al., must be evaluated with the understanding that non-paralyzed, conscious individuals subjected to immersion in water cooled to between 2°C and 12°C will shiver either until exhaustion or until the temperature of the skeletal muscle drops below 30°C at which point shivering is impossible.[151] Until either or both of these events happen core temperature will either transiently increase or will not decrease appreciably. Thus, use of these data to evaluate the efficacy of convective cold water immersion must subtract the first 10 to 20 minutes of core temperature data when shivering and elevated oxygen consumption are effectively maintaining homeostasis in the face of profound external cooling.[152]
From the graphic data it is very apparent when this point is reached, typically at about 15 minutes post-immersion, for the unclothed emaciated subject. At this point the cooling curve begins to decline markedly and within 5 minutes achieves a steady rate of descent of approximately 0.26°C/min to 28°C after which it slows appreciably to 0.13°C/min until cardiac arrest typically occurs at ~27°C. This slowing is probably due to deteriorating hemodynamic status (BP was typically 40 mmHg to 50 mmHg near the terminal portion of the cooling curve) and a decrease in the ∆T between subject core temperature and bath temperature. In one series of 7 subjects the mean time from immersion to ventricular fibrillation (VF) was 66 minutes with a mean rectal temperature at the point of VF of 26.94°C. The fastest rate of cooling observed was 0.36°C/min in malnourished females.
One potentially very important observation made by of Holzloehner, et al., was the lack of any difference in the rate of early core temperature drop between subjects immersed in stirred water baths at 2°C or 12°C. This would seem to imply that the rate limiting factor in cooling, at least to ~27°C, is not the volume of water flowing over the subject, nor the ∆T, but rather peripheral vasoconstriction resulting in decreased blood flow to tissues in contact with cold water. Stirring of the PIB water in excess of that required to achieve optimum heat exchange is also undesirable for the following reasons:
- Given the modest amount of stirring employed in the experiments of Holzloehner, et al., it is unlikely that movement of large volumes of water over the patient will increase the rapidity or efficiency of heat exchange.
- Excessive stirring may result in unnecessary heat exchange with surrounding air, and unnecessarily waste heat generated by stirring pumps.
- Smaller more efficient pumps will allow for the use of household alkaline or lithium batteries facilitating initiation of SCCD cooling in the field and during vehicular transport where 110VAC wall current is unavailable.
- Increasing the efficacy of external cooling further will require improvements to either or both cardiac output and peripheral perfusion by either pharmacologic and/or mechanical means.
It is important to consider that temperature was measured rectally in the subjects of these experiments as opposed to esophageally or tympanically and thus cardiac and brain core temperatures were probably 2- 4°C lower than those reported since rectal temperature is known to lag significantly behind esophageal temperature during induction of hypothermia during both external cooling and extracorporeal cooling.[153],[154],[155]
Third, comparison between subjects is difficult due to the lack of body mass or surface area measurements, or any determination of fat cover; this is a problem which must also be resolved in human cryopreservation cases to facilitate comparisons of the efficacy of cooling methods among cases.
Finally, quantification of hemodynamic status during cooling was not done. Blood pressure was measured only after the subject was removed from the cooling bath; not during active cooling. Similarly, measurement of cardiac output and peripheral and systemic vascular resistance were not techniques available at that time. However, it seems reasonable to presume that as the investigators reported, most subjects were maximally peripherally vasoconstricted. This can be inferred from the gruesome clinical descriptions contained in the data which report facial skin becoming immediately pale and then cyanotic after the first 45 to 50 minutes of cold water immersion (i.e., near the point of cardiac arrest).
The data also disclose the importance of cooling both the neck and the occiput of the head if the maximum rate of temperature descent was to be achieved, presumably reflecting both the rich perfusion of the scalp and the cooling of the high flow and relatively superficial jugular and carotid blood flows. Failure to cool the neck and occiput were sufficient to reduce core temperature drop by approximately 0.16°C/min.
The data contained in the Alexander Report were sufficiently convincing for the author to initiate a change in Alcor protocol from ice bag cooling to one employing cold water immersion using the PIB. This decision was financially costly and introduced serious new logistical hurdles. PIBs must be constructed, air transported and add hundreds of additional pounds of weight in water and ice which must be picked up and moved during Transport necessitating additional personnel.
Late in 1989 Fred Chamberlain, co-founder of Alcor, developed a prototype SCCD using an AC powered sump-type submersible pump to facilitate convective cooling. The SCCD was first used in 1990 on a profoundly chachectic patient with a mass of only 32.8 kg and virtually no fat cover.[156] This patient exhibited an extraordinarily good and sustained response to CPS maintaining excellent peripheral tissue perfusion and oxygenation throughout the entire 2.5 hours of Thumper supported CPS. The patient’s core cooling rate 0.41°C/min during the first 12 minutes of closed chest CPS and external cooling and of 0.33°C/min during the first 43 minutes of closed chest CPS and external cooling still appears extraordinary for cryopreservation patients; although it is consistent with the data for chachectic human concentration camp victims generated by Holzloehner, et al. In the future comparisons between patients can be better facilitated by using the patient’s Body Mass Index (BMI) as an objectifying comparison tool. BMI is calculated as follows:
BMI=KG/m2
The patient’s weight and height are required to calculate BMI. Thus, it will be critical to measure the patient’s weight in kg (either prior to cardiac arrest or upon arrival at the cryoprotective perfusion facility) and measure their height in cm prior to the start of cryoprotective perfusion.
 Figure 21: Skinfold caliper; both analog and digital versions are available.
Figure 21: Skinfold caliper; both analog and digital versions are available.
It may also be very useful to estimate body fat and more reliably determine subcutaneous fat cover by using a Skinfold Caliper. A Skinfold Caliper is a device which measures the thickness of a fold of skin with its underlying layer of fat. By measuring skin fold thickness at precise places on the thorax, abdomen and thigh a reasonably accurate measurement of total body fat can be obtained in a healthy individual. In the case of the cryopreservation patient the interest is not primarily in the accuracy of the tool in determining total body fat per se, but rather in the creation of a database of measurements which can serve as an objectifying guide to determining a patient’s degree of fat cover at the time of cardiopulmonary arrest.
Illustrated in the charts and tables below is the procedure for taking Skinfold Caliper measurements in both adult males and females:
Measuring Sites for Males
| Site | Direction of fold | Measurement |
| Thorax | Diagonal | Fold is taken ½ the distance between the anterior auxiliary line and nipple. |
| Abdomen | Vertical | Fold is taken vertical 2 cm lateral to the umbilicus. |
| Thigh | Vertical | Fold is lifted on anterior aspect of thigh midway between inguinal crease and proximal border of patella. Body weight is shifted to left foot. |
Table 2: Measuring Sites for Skincaliper Body Fat Determination:
Measuring Sites for Female
| Site | Direction of fold | Measurement |
| Triceps | Vertical | Fold is taken midway between the shoulder and elbow joint, on the center of the back of the arm. |
| Waist | Diagonal | Fold is taken diagonally above the iliac crest along the anterior auxiliary line. |
| Thigh | Vertical | Fold is lifted on anterior aspect of thigh midway between inguinal crease and proximal border of patella. Body weight is shifted to the left foot. |
Multiple regression equations exist for calculating the percentage of body fat from the Skinfold Caliper measurements depending upon age and sex (50-52). However, at this time the author believes that the selection of a particular algorithm is not very important. Because there is no typical cryopreservation patient, the consistent collection of reliable data and their reduction to arbitrary numbers which can be compared from case to case is the most important consideration.
Collection of BMI and Skinfold Caliper measurements from multiple patients combined with some objectification of the efficacy of CPS during transport will likely be the most useful tools for comparing various methods of cooling; both external and internal.
Below are the Jackson, et al., equations for calculation of percentage body fat in females and males:
FEMALES:
D = 1.0994921 – (0.0009929 x sum of triceps, suprailiac, thigh) + (0.0000023 x square of the sum of triceps, suprailiac, thigh) – (0.0001392 x age), based on a sample aged 18-55.
Jackson, et al. (1980) Generalized equations for predicting body density of women. Medicine and Science in Sports and Exercise, 12:p175-182.
MALES:
D = 1.10938 – (0.0008267 x sum of chest, abdominal, thigh) + (0.0000016 x square of the sum of chest, abdominal, thigh) – (0.0002574 x age), based on a sample aged 18-61.
Jackson, A.S. & Pollock, M.L. (1978) Generalized equations for predicting body density of men. British J of Nutrition, 40: p497-504.
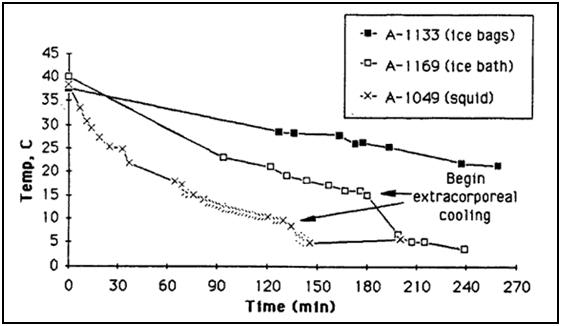 Figure 22: Actual cooling curves for three adult patients on HLR support, using ice bags, portable ice bath (PIB), and PIB plus SCCD cooling. Patient A-1133 weighed 56.8 kg, patient A-1169 weighed 57.3 kg, and patient A-1049 weighed 36.4 kg. As this data indicates, PIB cooling is approximately twice as effective as ice bag cooling. The spray cooling device (SCCD) increases the rate of cooling by yet another 50% over the PIB (roughly adjusting for differences in patient weights).
Figure 22: Actual cooling curves for three adult patients on HLR support, using ice bags, portable ice bath (PIB), and PIB plus SCCD cooling. Patient A-1133 weighed 56.8 kg, patient A-1169 weighed 57.3 kg, and patient A-1049 weighed 36.4 kg. As this data indicates, PIB cooling is approximately twice as effective as ice bag cooling. The spray cooling device (SCCD) increases the rate of cooling by yet another 50% over the PIB (roughly adjusting for differences in patient weights).
As can be seen in Figure 38, cooling rates with the PIB, even when using stirred water to maximize convective cooling; still result in unacceptably slow rates of cooling. To solve this problem four other methods of cooling, which are amenable to immediate, in-field application have been developed:
1) Use of chilled parenteral medications; bulk IV fluids given to provide cerebroprotection, such as buffers and hyperosmotic agents are administered (where possible) chilled to 1-2 oC.
2) Peritoneal lavage with 4 liters of Normosol-R™ chilled to 1-2 oC is used 2x as soon as feasible after pronouncement.
3) Colonic lavage with1 liter of Normosol-R™ chilled to 1-2 oC is used 4x, as an alternative or prior to peritoneal lavage, as soon as possible during CPS (total of 8 liters).
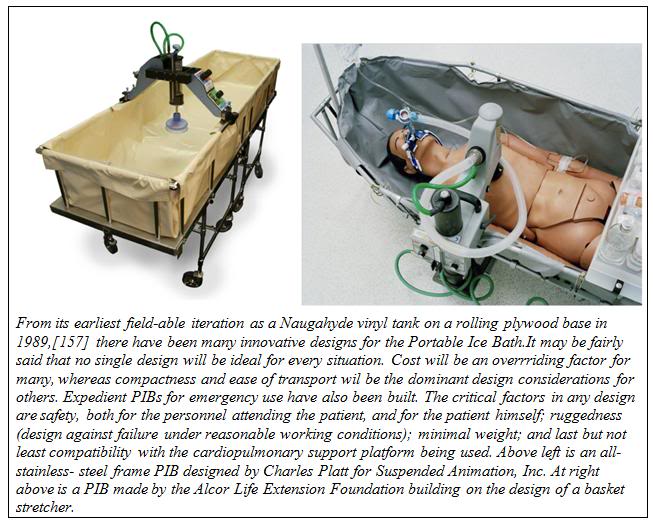
4) Liquid assisted pulmonary cooling (LAPC) with 4 liters of 3M FC-75™ perfluorocarbon is used 2x during CPS; the first as soon as possible after placement of an endotracheal tube, and the second 15 minutes following the first.
Detailed instruction on how to perform these adjunct procedures to external cooling are provided later in this Chapter.
References
References are given at the end of Part II of this article.
Footnotes
[1] The other two pillars are the information theoretic criterion of death and the assumed continued advance of technology and medicine.
[2] The Boston Circulatory Arrest Trial was carried out using alpha stat pH management which is no longer used by most centers for pediatric cardiac surgery involving DHCA.
[3] The Q10 rule is much abused in biology and a further exposition of the limitations on its use is presented in Appendix 1.
[4] A few qualifying remark are in order here. This statement holds only when the new therapeutic modality fits well within the existing biomedical paradigms, and social and ethical milieus. Semmelweis and antisepsis in the 18th Century were vigorously resisted whereas the sulfanilamide and penicillin were rapidly embraced 60 years later. Few clinicians question the potential of hypothermia, or the biological basis of its therapeutic action, to the extent it is currently understood. What is questioned is, ‘is it worth it?’

http://i293.photobucket.com/albums/mm55/mikedarwin1967/H23.jpg (the calipers) is a broken link.
Thanks, I’ll see if I can fix it. Thanks especially for telling me WHAT the URL was a picture of! — Mike Darwin