The Pathophysiology of IRI
While a wide range of post-insult interventions are currently being investigated in animal and clinical trials, and despite almost universal agreement that CI is a multifactorial insult, there has been little or no research aimed at developing a multimodal method of managing the multiple insults and compromises to brain metabolism that are known to occur.
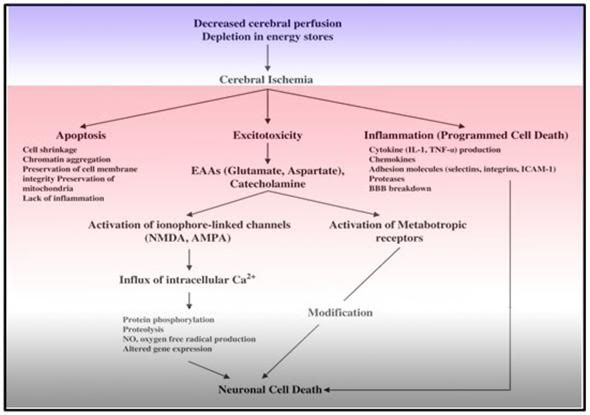 Figure 11: The big picture. The complex and interwoven cascade of events depicted above underscores the multifactorial nature of cerebral ischemia-reperfusion injury. Mono-modal drug treatment will not be effective in achieving neurosalvage in the presence of such a complex and interdependent pathophysiology.
Figure 11: The big picture. The complex and interwoven cascade of events depicted above underscores the multifactorial nature of cerebral ischemia-reperfusion injury. Mono-modal drug treatment will not be effective in achieving neurosalvage in the presence of such a complex and interdependent pathophysiology.
Before discussion of specific pharmacological and biophysical means for prevention and/or amelioration of ischemic injury are described in detail, it is desirable to briefly review the requirements for adequate cerebral perfusion and the basic mechanisms of cerebral ischemia-reperfusion injury (CIRI) as they are currently understood.
Normal cerebral blood flow (CBF) in the human is typically in the range of 45-50 ml/min/100g of brain tissue, as long as mean arterial pressure (MAP) is in the range of 60-130 mm Hg.53 When CBF falls below 20 to 30 ml/min/100g/brain tissue, marked disturbances in brain metabolism begin to occur, such as water and electrolyte shifts, with regional areas of the cerebral cortex experiencing failed perfusion.53 At blood flow rates below 10 ml/min/100g/brain tissue, sudden depolarization of the neurons occurs with rapid loss of cellular ionic homeostasis.54
 Figure 12: These eleven mechanisms are currently thought to underlie the injury seen after global cerebral ischemia-reperfusion. PARP activation and subsequent apoptosis, hyperglycemia and the post-resuscitation syndrome (which is characterized by deterioration of brain blood flows and electrical activity in conjunction with the development of multisystem organ failure) are unlikely to be of significance in the human cryopreservation setting since these events require prolonged (hours to days) of ongoing metabolism to present and mature.
Figure 12: These eleven mechanisms are currently thought to underlie the injury seen after global cerebral ischemia-reperfusion. PARP activation and subsequent apoptosis, hyperglycemia and the post-resuscitation syndrome (which is characterized by deterioration of brain blood flows and electrical activity in conjunction with the development of multisystem organ failure) are unlikely to be of significance in the human cryopreservation setting since these events require prolonged (hours to days) of ongoing metabolism to present and mature.
The Mean Arterial Pressure (MAP) necessary for cerebral viability following extended resuscitation efforts in dogs has been found to be above 40 mm Hg. It has been speculated that a minimum MAP of 45 to 50 mm Hg is required to preserve cerebral viability in man.55
Unfortunately, as is now well documented, conventional closed-chest CPR is generally incapable of consistently delivering MAPs >30 mm Hg in man.56 A clinical and laboratory evaluation of manual and mechanical CPR (using a pneumatically driven chest compressor and ventilator) demonstrated that only 3 of 15 acute cardiac arrest patients presenting for emergency room resuscitation had MAPs above 40 mm Hg.57 Even in the patient experiencing optimum machine-delivered CPR, lung compliance and blood gases tend to deteriorate rapidly during CPR, perhaps as a result of pulmonary edema secondary to high intrathoracic venous pressures.58
As the foregoing analysis makes clear, most SCA patients will suffer significant periods of cerebral anoxia, ischemia, or hypoperfusion resulting in currently irreversible brain damage. This effectively excludes these patients from receiving more effective cardiopulmonary support such as open chest CPR (OCCCPR),59or the use of extracorporeal circulation utilizing a membrane oxygenator60) as a bridge to definitive repair of their defective coronary circulation.
Mechanisms of Ischemic Injury
Early observations on the mechanisms of ischemic injury focused on relatively simple biochemical and physiological changes which were known to result from interruption of circulation. Examples of these changes are loss of high-energy compounds,61 acidosis due to anaerobic generation of lactate,62 and no-reflow due to swelling of astrocytes with compression of brain capillaries.63 Subsequent research has shown the problem to be far more complex than was previously thought and involving the action and interaction of many factors. 64
Biochemical Events
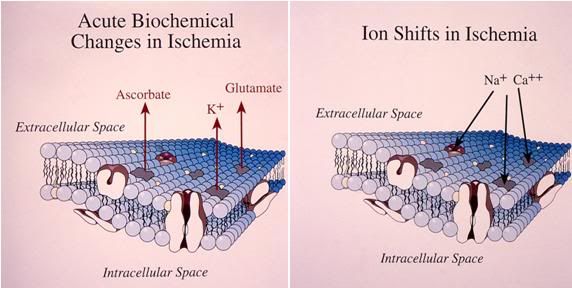 Figure 13: The acute biochemical changes which occur as a result of the interruption of cerebral perfusion consist primarily of ion shifts and the release of ascorbate and the excitatory neurotransmitters glutamate and aspartate. The movement of sodium (and accompanying water) into the intracellular compartment begins within 30 seconds of the onset of ischemia, as does the leakage on intracellular potassium into the extracellular space. The release of ascorbate and excitatory amino acids becomes of significance in the range of 3-5 minutes after the failure of perfusion.
Figure 13: The acute biochemical changes which occur as a result of the interruption of cerebral perfusion consist primarily of ion shifts and the release of ascorbate and the excitatory neurotransmitters glutamate and aspartate. The movement of sodium (and accompanying water) into the intracellular compartment begins within 30 seconds of the onset of ischemia, as does the leakage on intracellular potassium into the extracellular space. The release of ascorbate and excitatory amino acids becomes of significance in the range of 3-5 minutes after the failure of perfusion.
Within 20 seconds of interruption of blood flow to the mammalian brain under conditions of normothermia, the EEG disappears, probably as a result of the failure of high-energy metabolism. Within 5 minutes, high-energy phosphates have been exhausted (adenosine triphosphate (ATP) depletion65 and profound disturbances in cell electrolyte balance start to occur: potassium begins to leak rapidly from the neurons and glial cells, and sodium and calcium begin to enter the cells.66 Sodium (Na++) influx, particularly in the astrocytes, results in a marked increase in cellular water content.67 Concurrent with the rapid shift in ions there is marked leakage of ascorbate from the neurons and glial cells. Ascorbate, in the presence of free iron (see discussion under Free Radicals below) is one of the most potent pro-oxidants known.
Calcium
Normally, calcium (Ca++) is present in the extracellular milieu at a concentration 10,000 times greater than the intracellular concentration. This 10,000:1 differential is maintained by at least the following four mechanisms: 1) active extrusion of calcium from the cell by an ATP-driven membrane pump,68 2) exchange of calcium for sodium at the cell membrane driven by the intracellular to extracellular differential in the concentration of Na+ as a result of the cell membrane’s Na+/K+ pump,68 3) sequestration of intracellular calcium in the endoplasmic reticulum by an ATP-driven process,69 and 4) accumulation of intracellular calcium by oxidation-dependent calcium sequestration inside the mitochondria.70-72
The loss of cellular high-energy compounds during ischemia causes the loss of the Na+/K+ gradient which virtually eliminates three of the four mechanisms of cellular calcium homeostasis. This, in turn, causes a massive and rapid influx of calcium into the cell.73
Mitochondrial sequestration, the remaining mechanism, causes overloading of the mitochondria with calcium and diminishes their capacity for oxidative phosphorylation. Elevated intracellular Ca++ activates membrane phospholipases and protein kinases. A consequence of phospholipase activation is the release of free fatty acids (FFA’s) including the potent prostaglandin inducer, arachidonic acid (AA).
While reperfusion also restores ATP levels it will, as a consequence, allow active uptake of calcium by the mitochondria, resulting in massive calcium overload and destruction of the mitochondria. Ironically, a consequence of this Ca++ induced mitochondrial lysis is the creation of nano-domains of cytosol containing (transiently) very high concentrations of Ca++. This can result in further Ca++ (over)loading of nearby mitochondria.
The degradation of the plasma membrane by phospholipases almost certainly damages membrane integrity, further reducing the efficiency of calcium pumping. During the reperfusion phase after cerebral ischemia, calcium accumulates in mitochondria, and a burst of free radical formation occurs, conditions that favor the activation of the mitochondrial permeability transition pore ((PT) pore). As a result of this injury to the mitochondria cytochrome C is released, resulting in activation of at least three caspases. The caspases are one of the proteases in the cytochrome C pathway for triggering apoptosis. In vivo models of global and regional cerebral ischemia have demonstrated the release of large amounts of caspases 3, which is a known apoptosis-inducing protease. More recently, in vitro studies have demonstrated that other caspases are also being released. Attention has focused on caspase-9, which is released from isolated mitochondria on treatment with calcium, as possibly the primary signaling molecule for initiating apoptosis.
Similarly, in neuronal cell culture models, apoptosis-inducing agents trigger translocation of caspase-9 from mitochondria to the nucleus. Of greater relevance has been the very recent demonstration in vivo, in a model of transient global cerebral ischemia, that caspase-9 is released from the mitochondria. This study demonstrated accumulation of caspase-9 in the neuronal nuclei of hippocampal and other vulnerable neurons exhibiting early post-ischemic changes, preceding apoptosis. Loss of mitochondrial barrier function during neuronal injury from ischemia, and subsequent calcium influx, may therefore, play an important role in liberating certain caspases.74-77
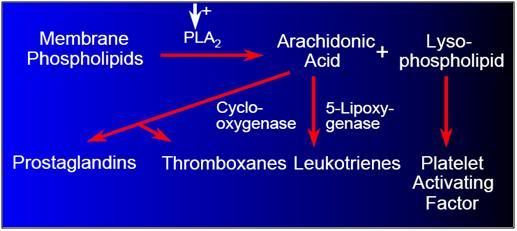
Figure 14: Damage to cell membrane components results in the production of pro-coagulant, pro-inflammatory, and vasoconstrictive ecisanoid molecular species, via the arachodonic acid cascade.
The production of AA as a result of FFA release causes a biochemical cascade ending with the production of thromboxane and leukotrienes,78,79 and the release of platelet activating factor.80 These compounds are profound tissue irritants,81 which can cause platelet aggregation,81clotting, vasospasm, and edema with resultant further compromise to restoration of adequate cerebral perfusion upon restoration of blood flow.
Free Radicals
During ischemia, the hydrolysis of ATP to AMP leads to an accumulation of hypoxanthine.82 Increased intracellular calcium enhances the conversion of xanthine dehydrogenase (XD) to xanthine oxidase (XO). Upon reperfusion and reintroduction of oxygen, XO may produce superoxide and xanthine from hypoxanthine and oxygen.83, 84 Even more damaging free radicals could conceivably be produced by the metal catalyzed Haber-Weiss reaction as follows.82, 83,84-87
O2- + H2O —-Fe3 ——> O2 + OH-+ OH-
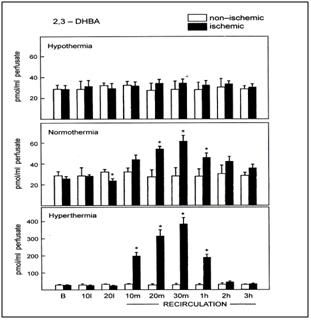 Figure 15: Hydroxyl radical trapping in forebrain of ischemic rats with 20 min of global forebrain ischemia followed by 2-hr of recirculation. Hypothermia = 30 to 30.5 degrees C, noromothermia = 36.5 to 37 degrees C, hyperthermia = 38.8 to 39 degrees C. Striatal microdialysis salycilate technique.
Figure 15: Hydroxyl radical trapping in forebrain of ischemic rats with 20 min of global forebrain ischemia followed by 2-hr of recirculation. Hypothermia = 30 to 30.5 degrees C, noromothermia = 36.5 to 37 degrees C, hyperthermia = 38.8 to 39 degrees C. Striatal microdialysis salycilate technique.
Iron, the transition metal needed to drive this reaction, is present in abundant quantities in bound form in living systems in the form of transferrin, hemoglobin the cytochromes and others. Anaerobic conditions have long been known to release such normally bound iron.88,89 Indirect experimental confirmation of the role of free iron in generating free-radical injury has come from a number of studies which have confirmed the presence of free-radical breakdown products such as conjugated dienes90 and low molecular weight species of iron.91 Paradoxically, the damaging effects resulting from the release of free iron from cytochromes, hemoglobin and possibly other iron-containing proteins during ischemia (iron delocalization) is likely greatly exacerbated by the endogenous antioxidant and free radical scavenger ascorbic acid (vitamin C). Ascorbate (reduced vitamin C) is an important enzyme cofactor, neuromodulator, and antioxidant that is stored at millimolar concentrations in the cytosol of cerebral astrocytes. Approximately 80% of the body stores of ascorbate are in the brain, and ascorbate is released in large quantities during ischemia.92 Despite its status as an antioxidant, in the presence of free iron, ascorbic acid is one of the most potent facilitators of the Fenton-Haber-Weiss reaction known, and its solo administration during reperfusion following ischemia is associated with increased neuroinjury.93, 94
During reperfusion and re-oxygenation, significantly increased levels of multiple free-radical species that degrade cellular and capillary membranes have been postulated: O2-, OH-, and free lipid radicals (FLRs). O2- may be formed by the previously described actions of XO and/or by release from neutrophils that have been activated by leukotrienes (see discussion below of the role of leukocytes in ischemia-reperfusion injury). Figure 1-16 is a table listing the free radicals currently known or suspected to be implicated in cerebral and systemic ischemia-reperfusion injury.
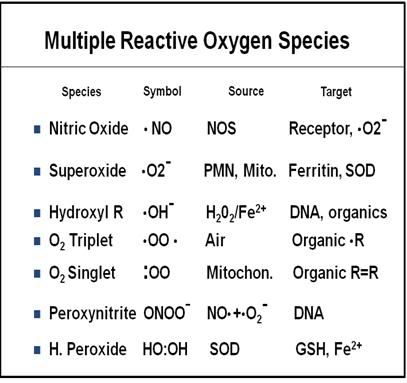 Figure 16: Multiple free radical species with diverse chemistries and stoichiometries are now known or suspected to mediate ischemia reperfusion injury (IRI). The implication of this is that multiple radical scavengers will be needed to treat those components of IRI which result from free radical activity. There is unlikely to be one “magic bullet” that quenches all of the offending radical species.
Figure 16: Multiple free radical species with diverse chemistries and stoichiometries are now known or suspected to mediate ischemia reperfusion injury (IRI). The implication of this is that multiple radical scavengers will be needed to treat those components of IRI which result from free radical activity. There is unlikely to be one “magic bullet” that quenches all of the offending radical species.
Nitric Oxide and Peroxynitrite
In 1992 Dawson, et al., 95 reported that nitric oxide (NO) a free radical gas synthesized from the amino acid l-arginine by the enzyme nitric oxide synthase (NOS), is produced in a wide range of tissues including, notably, the vascular endothelium, macrophages, polymorphonuclear white blood cells (PMLNs) and brain neurons and glial cells. These investigators also reported that NO was serving at least two functions; in the vascular endothelium it appears to be the long sought after “endothelial relaxing factor,” and in the brain it appears to function as a neurotransmitter, well as regulating cerebral blood flow and moderating inflammation.95, 96 To date three isoforms of NOS have been identified: the constitutively expressed neuronal (n-NOS) and endothelial isoforms (e-NOS) and the inducible isoforms (b-NOS).95,96 Neurons expressing n-NOS appear to be most densely concentrated in the cerebral cortex, hippocampus, and striatum; all areas that are selectively vulnerable to IRI.97,98 While it is apparent that NO is essential for cerebral vasoregulation and signal transduction, it is also clear that it is toxic when present at supraphysiologic levels. The direct cytotoxicity of NO is due to its inhibition of complexes I and II of the mitochondrial electron transport chain and it may inhibit other enzymes involved in high energy production, as well.96
PARP
An additional mechanism by which NO may cause injury during reperfusion is via the formation of the free radical peroxynitrite due to the reaction of NO and the superoxide anion98 Peroxynitrite generation, in turn, leads to the formation of other reactive oxygen species (ROS); hydroxyl free radicals, and nitrogen dioxide, the latter resulting in nitrosylation of tyrosine residues in proteins.
Peroxynitrite has been demonstrated both in vitro and in vivo to cause damage to both mitochondrial and nuclear DNA, resulting in activation of poly-(ADP-ribose)-polymerase (PARP), which is part of the cellular DNA repair system.99-101 Activation of PARP results in mitochondrial “futile cycles,” which consume from 30% to 60% of cellular high energy production, via massive depletion of NAD; resulting in direct (necrotic) cell death from the failure of high energy metabolism.102 So, while NO is essential for normal signal transduction, and is even protective against ischemia by virtue of its vasodilatory effects, when produced in large quantities during the ischemic interval it becomes both directly and indirectly toxic.
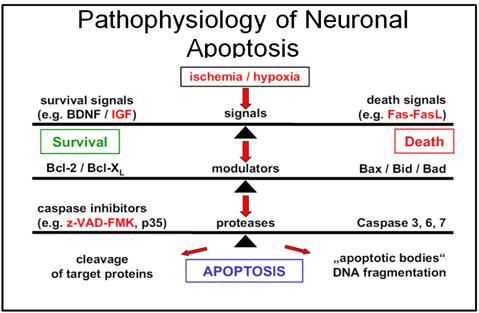 Figure 1-17: Cascade of signal transduction leading to apoptosis.
Figure 1-17: Cascade of signal transduction leading to apoptosis.
PARP activation triggers apoptosis in experimental models of sepsis and ischemia103,104 and increases mitochondrial metabolism during injury resulting in amplified oxidative stress and the generation of additional damaging free radicals and cytotoxic wastes. Free radicals, with or without PARP activation, may also be a primary initiator of nuclear factor kappa B (NFk B) production.105, 106
In GCIRI, constitutive NO activity, of both the endothelial and neuronal isoforms, is markedly increased as a consequence of NMDA, AMPA, and metabotropic glutamate receptor stimulation secondary to excitotoxicity (see “Excitotoxicity” below).107, 108 The activation of the metabotropic receptors causes a rise in intracellular Ca++, which in turn leads to sustained expression of NO by the microglia and other inflammatory cells (Ca++-independent inducible isoform) in the 24 hours following the ischemic event.109
References
53) Dearden, NM., Ischaemic brain. Lancet. 1985. 2(8449): p. 255-9.
53) ibid.
54) Hertz, L., Features of astrocyte function apparently involved in the response of central nervous system tissue to ischemia-hypoxia. J Cereb Blood Flow Metab. 1981. 1: p. 143-53.
55) McDonald, JL., Systolic and mean arterial pressures during manual and mechanical CPR in humans. Ann Emerg Med. 1982. 11(6): p. 292-5.
56) Del Guercio, L., A comparison of blood flow during external and internal cardiac massage in man. Circulation. 1965. Sppl 1: p. 171-80.
57) McDonald, JL., Systolic and mean arterial pressures during manual and mechanical CPR in humans. Ann Emerg Med. 1982. 11(6): p. 292-5.
58) Ornato, JP., et al., Measurement of ventilation during cardiopulmonary resuscitation. Crit Care Med. 1983. 11(2): p. 79-82.
59) Barsan, WG, Levy, RC., Experimental design for study of cardiopulmonary resuscitation in dogs. Ann Emerg Med. 1981. 10(3): p. 135-7.
60) Anstadt, MP., et al., Pulsatile reperfusion after cardiac arrest improves neurologic outcome. Ann Surg. 1991. 214(4): p. 478-88; discussion p. 489-90.
61) Macdonald, RL, Stoodley, M., Pathophysiology of cerebral ischemia. Neurol Med Chir. (Tokyo). 1998. 38(1): p. 1-11.
62) Kaplan, J., et al., Mechanisms of ischemic cerebral injury. Resuscitation. 1987. 15(3): p. 149-69.
63) Ames, A III., Cerebral ischemia II. The no-reflow phenomenon. Amer J Pathol. 1968. 52: p. 437-53.
64) Siesjo, BK., Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. J Neurosurg. 1992. 77(2): p. 169-84.
65) Siesjo, B., Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981. 1: p. 155-85.
66) Brooks, K.J, Kauppinen, RA., Calcium-mediated damage following hypoxia in cerebral cortex ex vivo studied by NMR spectroscopy. Evidence for direct involvement of voltage- gated Ca(2+)-channels. Neurochem Int. 1993. 23(5): p. 441-50.
67) McDonald, JL., Systolic and mean arterial pressures during manual and mechanical CPR in humans. Ann Emerg Med. 1982. 11(6): p. 292-5.
68) Carafoli, E., Curr Topics Memb Transport. 1978. 10: p. 151-216.
69) ibid.
70) Blaustein, M., The regulation of intracellular calcium in presynaptic nerve terminals. Proc NY Acad Sci. 1978. 307: p. 195-212.
71) Kumar, K., et al., Ultrastructural and ionic studies in global ischemic dog brain. Acta Neuropathol. 1987. 73(4): p. 393-9.
72) Fiskum, G., Mitochondrial damage during cerebral ischemia. Ann Emerg Med. 1985. 14(8): p. 810-5.
73) Kristian, T, Siesjo, BK., Calcium in ischemic cell death. Stroke.1998. 29(3): p. 705-18.
74) Raichle, ME., The pathophysiology of brain ischemia. Ann Neurol. 1983. 13(1): p. 2-10.
75) Krajewski, S., et al., Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci. U S A, 1999. 96(10): p. 5752-7.
76) Matsumoto, S., et al., Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1999. 19(7): p. 736-41.
77) Siesjo, BK., et al., Role and mechanisms of secondary mitochondrial failure. Acta Neurochir Suppl. 1999. 73: p. 7-13.
78) Rao, AM., et al., Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem Res. 1999. 24(10): p. 1225-32.
79) Boado, RJ., et al., Differential expression of arachidonate 5-lipoxygenase transcripts in human brain tumors: evidence for the expression of a multitranscript family. Proc Natl Acad Sci. 1992. 89(19): p. 9044-8.
80) Lindsberg, P.J, Hallenbeck, JM, Feuerstein, G., Platelet-activating factor in stroke and brain injury. Ann Neurol. 1991. 30(2): p. 117-29.
81) Aktan, S, Aykut, C, Ercan S., Leukotriene C4 and prostaglandin E2 activities in the serum and cerebrospinal fluid during acute cerebral ischemia. Prostaglandins Leukot Essent Fatty Acids. 1991. 43(4): p. 247-9.
82) Tien, M., An investigation into the role of hydroxyl radical in xanthine oxidase-dependent lipid peroxidation. Arch Biochem Biophys. 1982. 216: p. 142-51.
83) McCord, J., Oxygen derived free radicals in postischemic tissue injury. N Eng J Med. 1985. 312: p. 159-63.
84) Kleihues, K., Purine nucleotide metabolism in the cat brain after 1-hour of complete ischemia. J Neurochem. 1974. 23: p. 417-25.
84) Rhenchrona, S., Brain lactic acidois and ischemic cell damage: I Biochemistry and neurophysiology. J Cereb Blood Flow Metab. 1981. 1: p. 297-311.
85) Fridovich, I., Superoxide radical: An endogenous toxicant. Ann Review of Pharmacol Toxicol. 1983. 23: p. 239-57.
86) McCord, J., The superoxide free radical: Its biochemistry and pathophysiology. Surgery. 1983. 94: p. 412-14.
87) Tien, M., Comparative aspects of several models of lipid peroxidation systems. In Lipid Peroxides in Biology and Medicine, K., Yagi, ed., 1982. New York, Academic Press: p. 23-39.
88) Krause, GS., et al., Natural course of iron delocalization and lipid peroxidation during the first eight hours following a 15-minute cardiac arrest in dogs. Ann Emerg Med. 1987. 16(11): p. 1200-5.
89) Oubidar, M, et al., Ischemia-induced brain iron delocalization: effect of iron chelators. Free Radic Biol Med. 1994. 16(6): p. 861-7.
90) Nayini, NR., et al., Post resuscitation iron delocalization and malondialdehyde production in the brain following prolonged cardiac arrest. J Free Radic Biol Med. 1985. 1(2): p. 111-6.
91) Babbs, C., Role of iron ions in the genesis of reperfusion injury following successful cardiopulmonary resuscitation: Preliminary data and a biochemical hypothesis. Ann Emer Med. 1985. 14: p. 777-83.
92) Hillered, L., et al., Increased extracellular levels of ascorbate in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis. Neurosci Lett. 1988. 95(1-3): p. 286-90.
93) Santos, MS, et al., Synaptosomal response to oxidative stress: effect of vinpocetine. Free Radic Res, 2000. 32(1): p. 57-66.
94) Wie, MB, et al., Phenidone attenuates oxygen/glucose deprivation-induced neurotoxicity by antioxidant and antiapoptotic action in mouse cortical cultures. Neurosci Lett. 1999. 272(2): p. 91-4.
95) Dawson TM, Dawson, VL., Snyder SH. A novel neuronal messenger in brain: the free radical, nitric oxide. Ann Neurol, 1992. 32: p. 297–311.
96) Dawson TM, Dawson, VL., Nitric oxide: actions and pathological roles. Neuroscientist. 1995. 1: p. 7–18.
97) Samdani AF, Dawson, TM., Dawson VL., Nitric oxide synthase in models of focal ischemia. Stroke. 1997. 28: p. 1283–8.
98) Dalkara T, Moskowitz, MA., The role of nitric oxide in cerebral ischemia. In: Welch KMA, Caplan LR, Reis DJ, et al, editors. Primer on cerebrovascular disease. Philadephia: AcademicPress; 1997. p. 207–8.
99) Lo, EH, Bosque-Hamilton, P, Meng, W., Inhibition of poly(ADP-ribose) polymerase: reduction of ischemic injury and attenuation of N-methyl-D-aspartate-induced neurotransmitter dysregulation. Stroke,.1998. 29(4): p. 830-6.
100) Endres, M., et al., Ischemic brain injury is mediated by the activation of poly(ADP- ribose)polymerase. J Cereb Blood Flow Metab. 1997. 17(11): p. 1143-51.
101) Sun, AY. and JS., Cheng, Neuroprotective effects of poly (ADP-ribose) polymerase inhibitors in transient focal cerebral ischemia of rats. Chung Kuo Yao Li Hsueh Pao. 1998. 19(2): p. 104-8.
102) Tokime, T., et al., Enhanced poly(ADP-ribosyl)ation after focal ischemia in rat brain. J Cereb Blood Flow Metab. 1998. 18(9): p. 991-7.
103) Krajewski, S., et al., Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci. 1999. 96(10): p. 5752-7.
104) Doutheil, J., et al., Activation of MYD116 (gadd34) expression following transient forebrain ischemia of rat: implications for a role of disturbances of endoplasmic reticulum calcium homeostasis. Brain Res Mol Brain Res. 1999. 63(2): p. 225-32.
105) Hickenbottom, SL, Grotta, J., Neuroprotective therapy. Semin Neurol, 1998. 18(4): p. 485-92.
106) Szabo ,ST, de Montigny, C, Blier, P., Modulation of noradrenergic neuronal firing by selective serotonin reuptake blockers. Br J Pharmacol. 1999. 126(3): p. 568-71
107) Bhardwaj, A, Northington, FJ., Ichord, RN., et al. Characterization of ionotropic glutamate receptor-mediated nitric oxide production in vivo in rats. Stroke. 1997. 28: p 850–6.
108) Bhardwaj, A, Northington ,FJ., Martin, LJ., et al. Characterization of metabotropic glutamate receptor-mediated nitric oxide production in vivo. J Cereb Blood Flow Metab. 1997. 17: p. 153–60.
109) Samdani, AF, Dawson TM., Dawson VL., Nitric oxide synthase in models of focal ischemia. Stroke. 1997. 28: p. 1283–8.

Please send me Part 1 and Part 2 of this article. I find it very useful for understanding this topic of cerebral ischemia.
Regards
H
I have sent you the URL’s for all 4 parts of the article. — MD
This is a very useful and enlightening article.
Could you kindly send me the whole series of serbral ischaemia and other neuroscience topics you have written.Many thanks.
A
“How can we set up institutions to make cryonics understandable to people born in 2012, 2052, 2102, etc., who will have fundamentally different experiences and expectations about “the future” from the ones we grew up with? I see little to no interest in dealing with these problems in the cryonics community, much less discussing them.”
Mark, I think I saw an ad for the film MONEYBALL on Starz. It’s ostensibly a movie about baseball. I have no interest in baseball. If you want an answer to the question you pose (from me), watch MONEYBALL. If you’ve seen it before, watch it again; this time with cryonics explicitly in mind. Think about CI, Alcor, all of it – reflect on cryonics as it exists today as you watch the movie. If MONEYBALL isn’t on Starz yet, it is surely in Redbox, or available from Netflix.
Once you’ve watched it and had some time to think about it, let me know, and I’ll answer your question. — Mike Darwin
Here are the URLs to all four parts of the article:
http://wp.me/p1sGcr-2n
http://wp.me/p1sGcr-2w
http://wp.me/p1sGcr-3q
http://wp.me/p1sGcr-3u
You might also be interested in: http://chronopause.com/index.php/2011/02/23/does-personal-identity-survive-cryopreservation/
and:
http://chronopause.com/index.php/2011/05/30/going-going-gone/
http://chronopause.com/index.php/2011/05/31/going-going-gone%E2%80%A6-part-2/
http://chronopause.com/index.php/2011/05/31/going-going-gone-part-3/
Thanks so much for your interest.
Mike Darwin