By Mike Darwin
 Introduction
Introduction
Someone who wants to understand the critical technical, social, political or personal issues involved in cryonics may well turn to any of several FAQ’s (Frequently Asked Questions) sites hosted by the various cryonics organizations.[1],[2],[3] As someone who was responsible for writing some of the answers to the technical questions used in these FAQs, I was interested to find upon revisiting them for the first time in many years that they contained little more scientific and technical information than was available more than a decade ago. Of greater concern was the realization that in some cases, the rapid and sustained advances in neuroscience and cryobiology over the past two decades offer the possibility for far more definitively bounding answers to questions such as, “under what conditions is long term memory (LTM) and personality likely to survive (or not survive) cryopreservation, or be badly degraded?”
Beyond satisfying the intellectual curiosity of the public, these issues are a key component to informed consent for individuals considering cryopreservation for themselves, or for a family member, or other person for whom they may have the responsibility and authority to make such a decision. Furthermore, if it can be demonstrated that the biochemical and structural basis upon which memory and personality (personal identity) rest are degraded or destroyed by some cryopreservation techniques, while being preserved by others, then the issue of what treatment to choose within the sphere of human cryopreservation procedures becomes paramount.
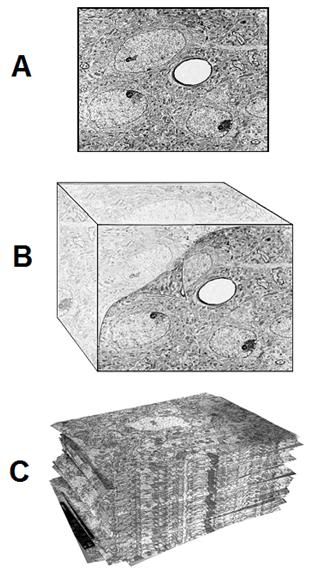 Figure 1: At top above (A) is a typical transmission electron micrograph (TEM) of cerebral cortex tissue at 10,000 x magnification. The slice from which this image was created was ~50 nanometers (nm) thick and was cut from a minute block of brain tissue (B) in which the water was replaced with a polymer that was subsequently plasticized. Generation of useful 3-dimensional images usually requires ~100 scanned, high resolution photographs (C) for input into the tomographic program.
Figure 1: At top above (A) is a typical transmission electron micrograph (TEM) of cerebral cortex tissue at 10,000 x magnification. The slice from which this image was created was ~50 nanometers (nm) thick and was cut from a minute block of brain tissue (B) in which the water was replaced with a polymer that was subsequently plasticized. Generation of useful 3-dimensional images usually requires ~100 scanned, high resolution photographs (C) for input into the tomographic program.
In the mid-1980s, neuroscientists studying the mechanics of how memory is encoded in the brain began to develop techniques precisely analogous to those used in medicine to create 3-dimensional images of tissue structure – but in this case, on the nanoscale as opposed to the macro-scale images produced from serial, uni-dimensional x-rays of tissue with Computerized Tomographic Scanning (CT-Scanning). Two techniques have been developed to allow such 3-dimensional nanoscale imaging of brain tissue: Electron Microscopic tomography[4, 5] and Ultrathin Serial Section Transmission Electron Microscopy (USSTEM).[6],[7] This latter technique consists of making ~ 100 serial sections of tissue (in this case of brain tissue), of 40 to 60 nm thickness and imaging them with conventional Transmission Electron Microscopy (TEM). The resultant images are captured on conventional high resolution photographic film[1], digitized using a standard flatbed scanner, and then subjected to computer processing using a standard PC running MS Windows to yield a 3-dimensional image which can be further manipulated, based on available software and the investigators’ objectives.[8] The process of USSTEM is shown in Figure 1. The serial images obtained using TEM are aligned and stacked, at which point software is used to render a 3-dimensional representation of the imaged tissue (Figure 2 and Figure 19).[9]
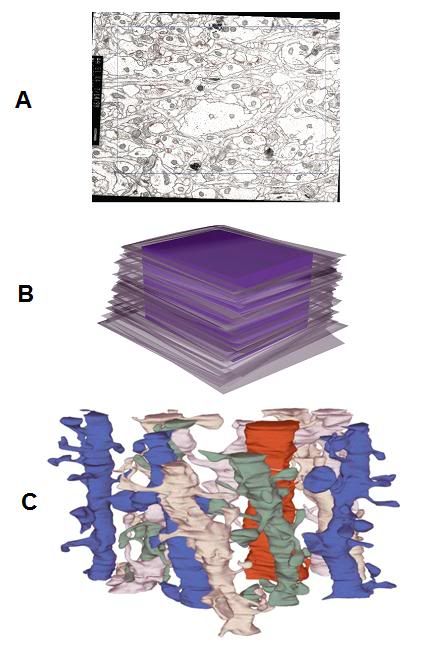 Figure 2: Sample volume reconstruction and analysis. Top, a section of the series (A) showing the sampling frame (blue) and identified synapses (red). The left and top edges of the frame are exclusion edges. A synapse that contacts an exclusion edge is marked with a yellow contour. Middle (B), the stack of serial sections for the aligned series is depicted in semi-transparent gray. After alignment, the sections form a volume with an irregular boundary due to the different transformations applied to each section. Inside this irregular volume, a rectangular prism or brick (purple) is defined as a reference volume for making density measurements. Finally, further processing (C) allows extraction of the desired reconstructed images from the sampled tissue block. In C, above, a set of 11 dendrite segments has been reconstructed from the serially TEM imaged tissue volume. The dendrites appear in the three-dimensional configuration that they have in the reconstructed volume. They are colored to help distinguish the individual segments and their synapse bearing spines. The red dendrite is a segment from an interneuron, as determined by the frequency and clustering of shaft synapses and the lack of mature-looking dendritic spines. [Fiala J.C., Harris K. M. (1999) Dendrite Structure. In Dendrites (eds. G. Stuart, N. Spruston, M. Häusser), Oxford University Press, in press.][9]
Figure 2: Sample volume reconstruction and analysis. Top, a section of the series (A) showing the sampling frame (blue) and identified synapses (red). The left and top edges of the frame are exclusion edges. A synapse that contacts an exclusion edge is marked with a yellow contour. Middle (B), the stack of serial sections for the aligned series is depicted in semi-transparent gray. After alignment, the sections form a volume with an irregular boundary due to the different transformations applied to each section. Inside this irregular volume, a rectangular prism or brick (purple) is defined as a reference volume for making density measurements. Finally, further processing (C) allows extraction of the desired reconstructed images from the sampled tissue block. In C, above, a set of 11 dendrite segments has been reconstructed from the serially TEM imaged tissue volume. The dendrites appear in the three-dimensional configuration that they have in the reconstructed volume. They are colored to help distinguish the individual segments and their synapse bearing spines. The red dendrite is a segment from an interneuron, as determined by the frequency and clustering of shaft synapses and the lack of mature-looking dendritic spines. [Fiala J.C., Harris K. M. (1999) Dendrite Structure. In Dendrites (eds. G. Stuart, N. Spruston, M. Häusser), Oxford University Press, in press.][9]
When consideration is given to the kinds of research cryonics organizations have funded over the past two decades,[10], [11] it is astonishing that they have not conducted in-house, nor commissioned extramural studies of this kind on brain tissue subjected to the cryopreservation techniques they are currently using (under both ideal, and the actual clinical conditions in which they are being applied). This is especially the case because the volume reconstruction system needed to perform this kind of imaging is freely available on-line as a Windows (2000, 95, 98, and NT) application called Serial EM (sEM) Align, and is straightforward to use. The sEM Align program was developed with the funding of the Human Brain Project and is available online at http://synapses.bu.edu./. [If cryonics organizations have retained existing tissue blocks from brain cryopreservation studies conducted in the past, these could easily be further sectioned, and the needed TEM photographs for tomographic reconstruction generated.]
Defining the Elements of Personal Identity – and it’s Destruction
What constitutes personal identity is a matter of considerable controversy and contention, and there may in fact be no single answer to the question that applies universally.[2] An excellent discussion of these issues, including a superb bibliography on the ‘problem’ of personal identity is available here: http://plato.stanford.edu/entries/identity-personal/.
Despite the uncertainty attending the definition of personal identity, it is possible to define and explore the biological and structural elements that comprise it. This is the case because only a limited number of biochemical and structural elements are candidates for encoding memory and personality, and it is now increasingly possible to image both this chemistry and structure.[12],[13],[14],[15],[16],[17],[18],[19, 20],[21] Similarly, without being able to succinctly define personal identity, we nevertheless find ourselves in the position of being able to determine when it is irretrievably lost by using the criterion of “information-theoretic death” as applied to the physical structures which encode and instantiate memory and personality. Information-theoretic death is the destruction of the human brain (or any cognitive structure capable of constituting a person) and the information within it to such an extent that recovery of the original person is theoretically impossible by any physical means. The concept of information-theoretic death (ITD) arose in the 1990s in response to the problem that as medical technology advances, conditions previously considered to be death, such as cardiac arrest, become reversible and are no longer considered to be death.[22] The criterion of ITD will be used to judge whether or not personal identity survives any given cryopreservation modality throughout this discussion.
“Information-theoretic death” is intended to mean death that is absolutely irreversible by any technology, as distinct from clinical death and legal death, which denote limitations to contextually-available medical care rather than the true theoretical limits of survival. In particular, the prospect of brain repair using molecular nanotechnology raises the possibility that medicine might someday be able to resuscitate patients even hours after the heart stops. The term “information-theoretic” is used in the sense of information theory.
Figure 3: DNA, the molecular starting recipe for constructing the individual.
The molecular foundation upon which personal identity is built is the genomes – the nuclear genome, which is comprised of genetic material from both parents – and the mitochondrial genome, which is inherited from the mother in the form of the mitochondria in the cytoplasm of the maternal oocyte. In particular, the instructions for constructing the individual that are present in the form of the nuclear DNA most powerfully contribute to the fundamental structural composition of the individual.
It is important to understand that DNA is a recipe, not a blueprint.[23] Nuclear DNA does not specify where every cell, let alone every molecule in the human body will be placed, just as a recipe for a cake does not specify where each gas bubble in the cake will be, what their precise relationship will be to each other, or even how many bubbles there will be. The proof of this in humans is seen in the case of identical twins. Twins do not share the same fingerprints, the same retinal (or cerebral) blood vessel arrangements, and where one twin is homosexual, there is only a 38% chance that the sibling male twin will be gay, and a 30% chance of shared sexual orientation in the case of female twins.[24],[25],[26],[27]
Additional determinants of brain and body structure occur during fetal development as a result of influence from the maternal biochemical environment; maternal circulating nutrient and hormone levels, harmful or beneficial maternal transmission of chemicals from the environment, and so on.
Similarly, there are biophysical influences from the environment during the growth and development of the child – and continuing through life, which may shape personal identity. Once maturation is reached, such influences are likely to be of less significance in shaping brain structure and biochemistry critical to memory and personality, although there are clearly exceptions to this rule.[3] During and after the completion of neurogenesis in the brain (~ 2 years of age)[28] the primary determinants of memory and personality will be experiential, and will take the form of long-term memories encoded in the molecular structure of the brain. It is the complex interaction of these “recorded” experiences with the hardware encoding and processing them, that constitute personhood. The nuclear genome continues to be of importance in that it is responsible for maintaining the neuronal machinery that encompasses the person, and it is even critical to the elaboration of proteins needed to modify brain structure in order to create and maintain long-term memories.[19]
Freezing Damage
In order to understand if personal identity survives cryopreservation, it is first necessary to understand the nature and extent of the damage that results from cryopreservation, via either freezing or vitrification, and under a range of clinically relevant conditions.
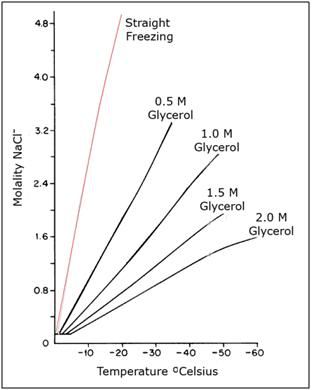
Figure 4: The water-sodium chloride-glycerol phase diagram above shows the enormous increase in salt concentration that cells are exposed to in the absence of cryoprotection (red line) and in the presence of increasing, but still modest concentrations of the cryoprotective agent (CPA) glycerol. Absent cryoprotection, there is a ~12 fold increase in the concentration of dissolved solids cells are exposed to by -20oC; the point at which no more water can be converted into ice upon further cooling.[29] The effect of this increase in the extracellular solute load on cell volume is shown in Figure 5, below.
A typical cartoon rendering of the effects of freezing at slow to moderate rates is shown in Figure 5, below. This scenario is accurate in that it correctly shows that under these conditions, ice forms first outside of cells, and the ice crystals consist of pure water.[30] Ice begins forming outside of cells rather than inside of them for at least two reasons. First, the concentration of dissolved materials in the water inside cells is much higher than that present in the water outside of the cells (in the extracellular space). These concentrated materials, primarily salts, such as phosphates and potassium chloride, exert a small amount of antifreeze activity, depressing the freezing point of the intracellular milieu about a degree Celsius (C) below that of the extracellular fluid.[31, 32]
The second factor in play is that for ice to begin forming at temperatures at or just below 0oC, it is necessary for molecules known as nucleators to be present.[33] Nucleators, or nidi as they are sometimes called, mostly consist of bacterial proteins and poorly characterized inorganic molecules, and serve as a template for ice growth to begin. This has the effect of raising the freezing point of water from -40oC to 0oC. It is believed that the intracellular space does not contain these nucleating agents.[34]
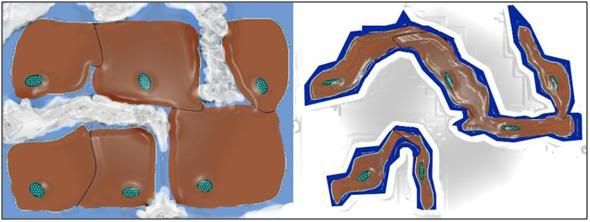 Figure 5: Typical representation of how freezing proceeds in cells and tissues. Ice begins forming outside cells, forming crystals of pure water. The salts and other solids that were formerly dissolved in the crystallized water are forced into a progressively smaller volume of unfrozen solution. This increase in the concentration of solids dissolved in the extracellular fluid osmotically extracts water from the cells, causing them to shrink. At ~ -20oC no further water can be converted into ice and the interior of the cells remains in an unfrozen state – a highly concentrated solution of cell proteins and salts, from both inside and outside the cells. With further cooling this electrolyte gel will be converted to a crystal free glass at ~ -100oC
Figure 5: Typical representation of how freezing proceeds in cells and tissues. Ice begins forming outside cells, forming crystals of pure water. The salts and other solids that were formerly dissolved in the crystallized water are forced into a progressively smaller volume of unfrozen solution. This increase in the concentration of solids dissolved in the extracellular fluid osmotically extracts water from the cells, causing them to shrink. At ~ -20oC no further water can be converted into ice and the interior of the cells remains in an unfrozen state – a highly concentrated solution of cell proteins and salts, from both inside and outside the cells. With further cooling this electrolyte gel will be converted to a crystal free glass at ~ -100oC
Once ice formation begins, as previously stated, it forms crystals of pure water, and this means that solids that were previously dissolved in the extracellular water being converted into ice are excluded from the ice crystals, and become dissolved in the progressively smaller volume of water that remains unfrozen. This has the effect of increasing the concentration of dissolved salts and proteins present outside of the cells, and as a result, water is removed from the cells by the increased extracellular osmotic pressure generated by the rising concentration of salts.[35],[36] As the temperature is reduced, and progressively more ice forms, more and more water is osmotically extracted from the cells until an equilibrium state is reached, and all the ice that can form has done so.[37] The result is what you see at right in Figure 5, above: severely dehydrated, shrunken cells surrounded by extracellular ice.[38] The interiors of such cells contain such highly concentrated salts and proteins that ice cannot form there. As cooling proceeds, eventually, at a temperature of ~ -100oC, this very viscous electrolyte gel that now comprises the intracellular space transitions from a liquid state to a solid state – in this case to a non-crystalline solid, a glass.[29]
Chemicals that provide cryoprotection are typically antifreeze compounds that are virtually identical in their action to that of ethylene glycol and propylene glycol, the chemicals used in automobile radiators, and to winterize plumbing in recreational vehicles to protect them against freezing damage.[36],[35],[39] These antifreeze compounds work not only by decreasing the point at which a mixture of the agents and water will freeze, but also by reducing the amount of ice that forms when freezing does occur.[40] They do this by two mechanisms: a) by the ‘bulk effect’ of taking up so much space in the solution that they physically interfere with the interaction of water molecules with each other, by providing a kinetic obstacle, and b) by so strongly hydrogen bonding to water that they prevent the water from continuing to be converted into ice, once a certain amount of ice has been formed. These mechanisms of action are termed “colligative cryoprotection.”[36],[35],[41] Instead of the entire volume of solution freezing, only part of it does, whilst the rest becomes a thick, un-freezable liquid that, upon further cooling, solidifies into a crystal-free and molecularly immobile glass (see Figure 6).[42] The more of the colligative cryoprotectant molecule(s) present in the solution (or in the cells or tissues), the less ice is formed (Figure 7).[36],[35]
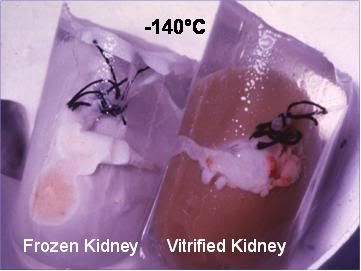 Figure 6: At left a rabbit kidney that has been frozen following treatment with ~ 40% cryoprotectant agents. The kidney was submerged in solution that did not have enough cryoprotective agents present to allow it to vitrify. The kidney has a chalky, opaque appearance due the presence of large amounts of ice in the tissue. At right is a kidney that has been perfused and equilibrated with sufficient cryoprotectant to allow cooling to -140oC with no ice formation. Because this kidney has no ice crystals in it to refract light, it remains translucent and appears unfrozen – which is in fact the case – even though it has been converted to a solid, glassy state.[43]
Figure 6: At left a rabbit kidney that has been frozen following treatment with ~ 40% cryoprotectant agents. The kidney was submerged in solution that did not have enough cryoprotective agents present to allow it to vitrify. The kidney has a chalky, opaque appearance due the presence of large amounts of ice in the tissue. At right is a kidney that has been perfused and equilibrated with sufficient cryoprotectant to allow cooling to -140oC with no ice formation. Because this kidney has no ice crystals in it to refract light, it remains translucent and appears unfrozen – which is in fact the case – even though it has been converted to a solid, glassy state.[43]
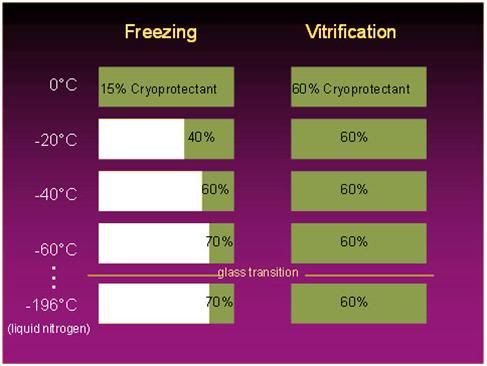
Figure 7: The fraction of a solution that is converted to ice upon freezing is a function of the amount of colligative cryoprotectant agent (CPA) present, as shown at left, above: the higher the CPA concentration, the smaller the fraction of the solution that is converted to ice at any given temperature. By contrast, in cryopreservation by vitrification the concentration of cryoprotectants is sufficiently high to prevent any ice formation during cooling, regardless of how low the temperature of the solution is cooled to. [Graphic courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
If enough antifreeze chemicals are present in the solution, no ice forms, and the solution is said to be vitrified (converted into a glassy state) if it is cooled below its solidification point.[44] Since crystallization does not accompany solidification of solutions when they vitrify, the point at which the solution transitions from a liquid to a solid state is called the Glass Transition Point, which is abbreviated Tg. The difference between vitrified and frozen tissue is readily apparent to the naked eye, as can clearly be seen in Figure 6, above.[43]
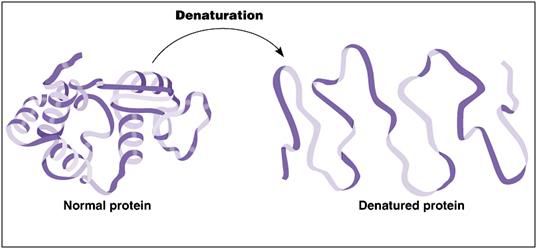 Figure 8: A ribbon model depicting the kind of conformational changes typically seen in protein denaturation.
Figure 8: A ribbon model depicting the kind of conformational changes typically seen in protein denaturation.
The obvious advantage that vitrification enjoys over freezing is the absence of ice formation. Because the molecules that comprise the solution both inside and outside the cells remain in an unorganized state that is virtually indistinguishable from its aqueous state, there is no mechanical injury to cells due to ice growth dehydrating and compressing them – and tearing, or otherwise degrading the connections between cells. The cells are also spared the many fold increase in intra- and extra-cellular ion (salt) concentration. This latter advantage is important, because one way in which cells are injured by freezing is at the molecular level. Many of the proteins that comprise the workhorses of cellular activity, including enzymes, DNA, RNA and the structural proteins critical to the cell maintaining its shape and membrane integrity, require the presence of water to maintain their functional structure.[45]
The water normally suspending and surrounding these proteins provides “conformational support” and is essential to many proteins maintaining their functional shape. When too much water is removed from the medium suspending proteins, the proteins may become denatured. What this means is that that the string of amino acids that make up the backbone of the protein can become unstable, and lose its necessary folding configuration, as seen in Figure 8, above. Since a protein is made up of a “bead work” of amino acids forming a long chain, it is often possible for that chain to be folded, or configured, in different ways. Unfortunately, usually only one folding configuration allows the protein to function in the lock and key fashion it requires in order to function properly – or at all.
When very high concentrations of salts or cryoprotective chemicals replace the native cellular water, protein can become completely denatured (Figure 8), or partially denatured. Sometimes this is reversible, but most often it is not, and the only solution to protein denaturation is for the cell to synthesize a replacement protein. However, if all of a species of protein that is essential to cellular metabolism is rendered inactive by denaturation, then the cell does not have the opportunity to restart metabolism, and thus replace the damaged protein(s).
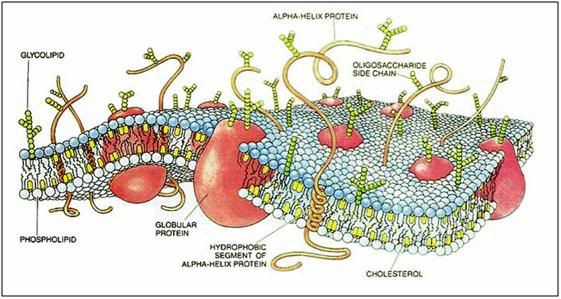 Figure 9: Cartoon of cell membrane structure.The plasma membrane is composed of a lipid bilayer made up primarily of phospholipids with cholesterol interdigitated in the interior of the phospholipid bilayer. A large variety of proteins, carbohydrates, and glycolipids are studded into, or decorate the membrane and serve as receptors, signal transducers, molecular transporters, pores, ion channels and pumps. At physiological temperatures the membrane lipids exist in a disordered, highly liquid state which is converted to a more organized gel state upon cooling to ambient temperature (~ 20oC). Further cooling can result in profound phase change in the membranes lipids resulting poration of the membrane and/or the redistribution or exclusion of embeded proteins and other structures essential to normal cell function.[46]
Figure 9: Cartoon of cell membrane structure.The plasma membrane is composed of a lipid bilayer made up primarily of phospholipids with cholesterol interdigitated in the interior of the phospholipid bilayer. A large variety of proteins, carbohydrates, and glycolipids are studded into, or decorate the membrane and serve as receptors, signal transducers, molecular transporters, pores, ion channels and pumps. At physiological temperatures the membrane lipids exist in a disordered, highly liquid state which is converted to a more organized gel state upon cooling to ambient temperature (~ 20oC). Further cooling can result in profound phase change in the membranes lipids resulting poration of the membrane and/or the redistribution or exclusion of embeded proteins and other structures essential to normal cell function.[46]
Of course, cells are not just made up of proteins; they are also composed of lipids (fats) and carbohydrates. The lipids, in combination with the proteins, constitute the primary structural elements of the cells: the intracellular organelle membranes and the plasma membrane that separates the intra- from the extra-cellular spaces. The cellular membranes are the walls, floors and tanks that make up the organized structure of the cell. However, far from being passive dividers or containers, the cellular membranes are incredibly complex molecular machines which are studded with proteins that perform a vast range of functions, from the mundane to the nearly miraculous (Figure 9).[47],[48] The cell membrane selectively controls the passage of nutrients, ions, water and a nearly endless array of signaling molecules into and out of the cell. Embedded in the membrane are structural support elements, and complex protein and protein-lipid complexes that act as molecular gatekeepers and message senders and receivers, facilitating communication between the cell and its environment.
Vitrification Damage
Both freezing and vitrification can damage cell membranes and cell proteins in the same ways. The antifreeze chemicals that comprise vitrification solutions are not water, and the very property that makes them useful in tightly binding water and preventing it from forming ice, makes then unable to fully pinch hit for water biochemically.[4] As a consequence, replacement of too much of the cellular water content with cryoprotectants in order to secure vitrification upon cooling, can result in protein denaturation.[49] Additionally, the colligative compounds used to achieve vitrification have the potential to solubilize or dissolve membrane components,[41] and they may also destabilize the lipid bilayer that comprises the cell membranes – or further facilitate its disturbance during cooling.
As seen in Figure 9, the cell membrane is composed of a laminar bilayer of phospholipids and cholesterol, studded with proteins, glycolipids and carbohydrates which serve as regulatory portals, signal transducers, and transporters for wastes and for molecules essential for metabolism and structural maintenance. This bilayer is normally lamellar – a smooth arrangement of phospholipids with the hydrophilic polar heads pointing out and the hydrophobic portion forming the core of the membrane bilayer. These phospholipids and cholesterol which comprise the membrane are mostly present in the liquid state at body temperature. However, these lipids, independent of the cellular water, undergo freezing, or phase change when cooled. Many cell membrane lipids freeze at or near room temperature, and almost all cellular lipid species are frozen at high subzero temperatures (i.e., above -20oC).[50],[51]
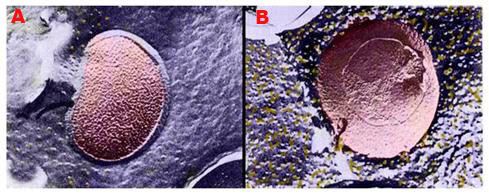
 Figure 10: Above, top, shows a false-color rendering of the normal configuration of membrane lipids and the membrane protein sodium-potassium-ATPase in a bacterial cell membrane. The membrane exhibits a smooth, lamellar character, and there are only a few aggregated and displaced particles of protein evident (yellow granules). In B, at right above, there is evidence of an alteration in membrane structure after the cell has been incubated at ~ -6oC for 1 hour in the presence of 20% w/v dimethylsulfoxide (Me2SO4). The membrane has developed a pebbly appearance and there are many extruded granules of protein on the membrane surface.
Figure 10: Above, top, shows a false-color rendering of the normal configuration of membrane lipids and the membrane protein sodium-potassium-ATPase in a bacterial cell membrane. The membrane exhibits a smooth, lamellar character, and there are only a few aggregated and displaced particles of protein evident (yellow granules). In B, at right above, there is evidence of an alteration in membrane structure after the cell has been incubated at ~ -6oC for 1 hour in the presence of 20% w/v dimethylsulfoxide (Me2SO4). The membrane has developed a pebbly appearance and there are many extruded granules of protein on the membrane surface.
The lower illustration (above) is a computer rendering of various lipid phase transitions in a model system (Langmuir trough), some of which result in perforations of the normally lamellar membrane structure.
Lipid phase change can result in a variety of alterations to membrane structure, and thus function.[52], [53] Reorganized lipids in the membrane may open up holes or pores which allow the leakage of water and ions into or out of the cells.[54] Cooling, independent of freezing, can also irreversibly alter the structure of glycolipids and lipid-protein complexes, rendering them inactive. Phase change in the membrane can also result in the precipitation, relocation, or extrusion of membrane proteins critical to cellular function, as seen in Figure 10.[55] Figure 10A shows the normal configuration of a cell membrane after rapid cooling to a stable, deep subzero temperature. The smooth lamellar character of the membrane is conserved, and very few of the particles (yellow) of sodium-potassium-ATPase protein are seen adjacent to the embedded lipoprotein structure (pink) in the membrane. In Figure 10B it is evident that the membrane has lost its smooth lamellar character and has undergone phase change. There is extensive relocation of the sodium-potassium-ATPase from the interior domain of the membrane to the surface, as well as aggregation of the protein into visible particles.
TEMs of membranes subjected to cooling to below the phase transition point of the lipids comprising them show the presence of long cylinders, which resemble the inverted hexagonal phase formed by some lipid-water dispersions under conditions of extreme dehydration. In this phase, water is found in long narrow cylinders on a hexagonal array, wherein each cylinder is surrounded by the hydrophilic moiety of the lipids. This morphology creates pores in the membrane forming a semipermeable barrier. A variety of other topological alterations in membrane structure have also been reported as a result of cooling.[56],[46],[57] Lateral phase separations may also occur in the fluid state, and under conditions of low hydration, whether from the removal of water from the cellular milieu by ice formation, or as a consequence of replacement of a large fraction of the cellular water with cryoprotectants, islands of protein free membrane are observed in TEMs. Further, phase separation of membrane components may occur, which result in one phase which is enriched in highly hydrating lipid species, and another which is enriched in weakly hydrating lipid species. The significance of this alteration is that the inverted hexagonal phases can most easily be formed by weakly hydrating lipids in the absence of protein – and the change from the lamellar to the hexagonal phase compromises the relative impermeability of the membrane to ions, and even to larger molecular species.[58]
The longer a cell is held at temperatures intermediate to the freezing points of membrane lipids, and stable, very low subzero temperatures (i.e., below – ~50oC), the more likely lipid phase change is to occur, and the more extensive such changes will likely be.[59] The chemical composition of the medium inside and outside the cells may also act to either stabilize, or destabilize membrane lipids, with respect to phase change.[60] So, while these changes can and do occur in the course of freezing, they are likely to be more extensive and more biologically significant in organs (or other large systems) subjected to vitrification, where there is necessarily prolonged exposure to high concentrations of cryoprotectants in the presence of high subzero temperatures, before the system is cooled to sufficiently to arrest these adverse alterations in membrane morphology.[61],[62]
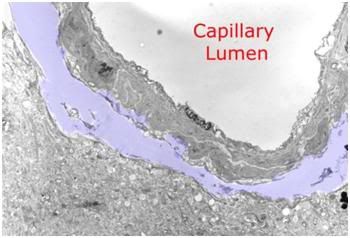 Figure 11: Peri-capillary tear in the parenchyma of the cerebral cortex as a result of hyperosmotic dehydration secondary to cryoprotective perfusion prior to vitrification. The space resulting from the dissection of the neuropil from the capillary basement membrane has been colored with a light purple tint.
Figure 11: Peri-capillary tear in the parenchyma of the cerebral cortex as a result of hyperosmotic dehydration secondary to cryoprotective perfusion prior to vitrification. The space resulting from the dissection of the neuropil from the capillary basement membrane has been colored with a light purple tint.
There is also osmotic injury which occurs during brain cryopreservation employing currently available vitrification techniques. The permeability of cell membranes to most cryoprotectants is very slow when compared with water. As a consequence, these osmotically active CPAs extract water from the intracellular space more rapidly than they can equilibrate with it, given the constraints imposed by the toxicity of these agents: namely, that they must be introduced at low temperatures (sometimes well below 0oC) and that the exposure time at these relatively high temperatures must not be too long. The relative impermeability of colligative cryoprotectants to brain tissue is not, as is commonly misunderstood, a function of the blood brain barrier (BBB). Most colligative CPAs freely cross the BBB because they are lipid soluble. Rather, it is due to the kinetics of water and CPA movement across the individual brain cell membranes. The multiply membrane wrapped myleinated axons that comprise the white matter pose a special diffusion barrier, in this respect. The cerebral dehydration that results from perfusion with high molarity CPA solutions causes occasional peri-capillary tears (Figure 11) as well as infrequent small tears within the brain parenchyma.
Fracturing
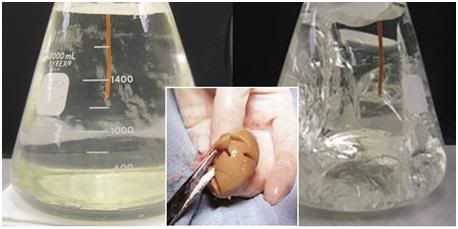 Figure 12: At left, above, is a flask of vitrification solution cooled to its solidification point of ~ -135oC. The solution is unfrozen and solid – a glass. At right, is what happens when the solution is further chilled to ~ -160oC; the solution has extensively fractured. The center insert shows a rabbit kidney equilibrated with 4M glycerol and then frozen to -196oC. It has extensively fractured, as well. The degree of fracturing seen in the photos above is worst case. Since the time this phenomenon was discovered, considerable work has been done to reduce the number and severity of fractures. This has been accomplished by slowing the rate of cooling during and after Tg, as well as providing for a period “stress relaxation” by annealing, or holding at a temperature near Tg. [Solution in flasks images are courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
Figure 12: At left, above, is a flask of vitrification solution cooled to its solidification point of ~ -135oC. The solution is unfrozen and solid – a glass. At right, is what happens when the solution is further chilled to ~ -160oC; the solution has extensively fractured. The center insert shows a rabbit kidney equilibrated with 4M glycerol and then frozen to -196oC. It has extensively fractured, as well. The degree of fracturing seen in the photos above is worst case. Since the time this phenomenon was discovered, considerable work has been done to reduce the number and severity of fractures. This has been accomplished by slowing the rate of cooling during and after Tg, as well as providing for a period “stress relaxation” by annealing, or holding at a temperature near Tg. [Solution in flasks images are courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
A type of injury common to cryopreservation by both freezing and vitrification is fracturing injury.[42, 63],[64],[65]When cryoprotective solutions, or organs loaded with them are cooled to significantly below the glass transition point of the CPA-water solution, internal stresses begin to build up.[66],[67] Since CPA-water glasses are inherently weak (and most tissues are little stronger), the result is cracking, or fracturing of the solution, or of the organ, as seen in Figure 12, above. Various protocols of controlled, ultra-slow cooling, and of annealing by holding the temperature steady for an interval at or near Tg, help to reduce the number and size of fractures, but no technique has yet been identified which eliminates them. Even storing just below Tg still causes some fracturing to occur.[42, 68]
Biological versus Electromechanical Machines
It is probably no accident that a large fraction of the people recruited to cryonics since its inception have been engineers, mathematicians, computer scientists, programmers, and, in general, physical science types.[5] One likely reason for this is that there is a fundamental difference in the way biological ‘machines,’ and electromechanical machines are structured and operate. Almost all electromechanical systems may be fairly described as solid state – even those in existence before the era of “modern electronics” and the advent of the transistor. By solid state, what is meant here is that virtually all their components are, literally, solid at their normal operating temperatures.
 Figure 13: Living cells are not electro-mechanical machines comprised of solid-state, rigid parts which fracture, or break into discrete, easily identifiable pieces of debris when mechanically damaged.
Figure 13: Living cells are not electro-mechanical machines comprised of solid-state, rigid parts which fracture, or break into discrete, easily identifiable pieces of debris when mechanically damaged.
A consequence of this is that it is possible to take a 21st Century mobile phone, or even a 17th century mechanical clock, and hit them with a sledgehammer, and the end result would broadly be the same: a collection of solid pieces of varying sizes and shapes that just sit there – and of course, neither the phone nor the clock would be functional. However, all the pieces would continue to exist unchanged – and they would retain their individuality and unique identity (Figure 12). And such would be the case indefinitely, as long as they are protected from the elements (moisture, oxidation, corrosion) and are kept at a temperature below their melting or combustion points.
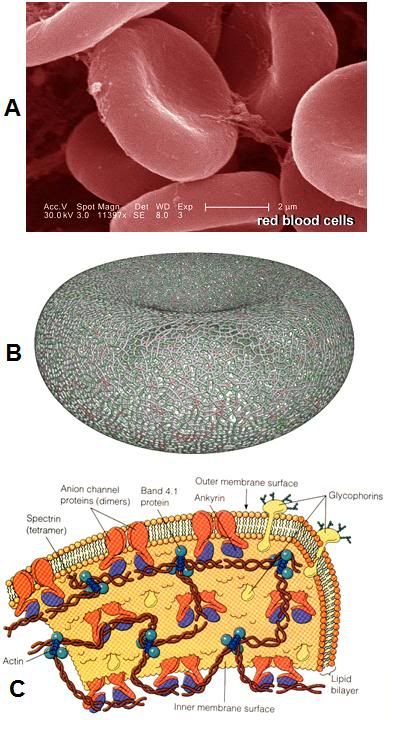 Figure 14: Even the simpler cells in the human body are surprisingly complex, as is the case with the red blood cells (RBCs), as seen above. At left, (A) is an electron micrograph of RBCs with a 2 micron scale bar present – each RBC is ~ 8 microns in diameter. Red cells lack nuclei and the complex interior structure of other somatic cells; however their structure is still enormously complex. As can be seen in (B) at left, the cytoskeleton, the molecular framework that gives the RBC its unique biconcave shape and deformability, is comprised of an intricate web work of proteins. The membrane itself (C) is studded and interdigiated with a wide array of complex proteins arranged into pores, pumps, and signaling devices.[48]
Figure 14: Even the simpler cells in the human body are surprisingly complex, as is the case with the red blood cells (RBCs), as seen above. At left, (A) is an electron micrograph of RBCs with a 2 micron scale bar present – each RBC is ~ 8 microns in diameter. Red cells lack nuclei and the complex interior structure of other somatic cells; however their structure is still enormously complex. As can be seen in (B) at left, the cytoskeleton, the molecular framework that gives the RBC its unique biconcave shape and deformability, is comprised of an intricate web work of proteins. The membrane itself (C) is studded and interdigiated with a wide array of complex proteins arranged into pores, pumps, and signaling devices.[48]
Biological systems are not solid state devices, and they operate in very different ways from such instruments. The core of biological systems is the membrane; and, as previously noted, membranes in living systems are not just passive walls or “compartment makers.” They are the engines of chemistry and action in living systems (Figures 9, 14 and 16). They have enormous complexity and they derive a great deal of their unique ability to function as living systems from their liquidity and plasticity.[48] A physical analogy in the everyday macro-world would be that of soap bubbles (Figure 15). Cell membranes are very much like soap bubbles and they behave in broadly similar ways when stressed. If a cell is osmotically stressed by shrinking or swelling it too much, it does not behave like a glass sphere and shatter into discrete pieces, which can be collected and reassembled. Rather it buds and ‘blebs’ and behaves like what it is, a liquid. And if it is stressed enough, it comes apart into little droplets and into smaller ‘cells;’ blebs and vesicles that have formed from the original membrane (Figure 17).

Figure 15: The soap bubble is a tolerable working analogy to the cell membrane – both the plasma membrane that encases the cells – and the intracellular membranes that bound the cellular organelles, such as the nuclei and the mitochondria, exhibit the same liquid based, space enclosing behavior.
Before disintegration or blebbing of the cell membrane occurs in response to stress (e.g., osmotic, temperature reduction, dehydration, chemical destabilization), the structure of the membrane can undergo reorganization in many ways, and the proteins embedded in the membrane may be rearranged as well. This is true not just for the plasma membrane that encloses the cell, but also for the membranes that comprise the cellular organelles. In fact, it is just such a rearrangement of mitochondrial membrane structure that underlies some of the damage that occurs in ischemia.[69] [6] Freezing causes enormous biophysical stress to the plasma membrane, and to organelle membranes, and some of the response to this stress is to radically alter membrane structure.
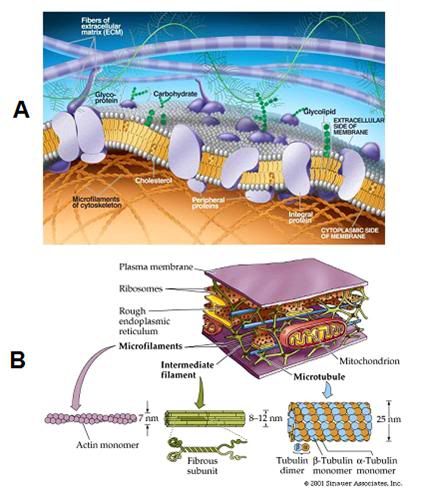 Figure 16: Far from being simple, passive, devising walls, cell membranes are complex biological machines consisting of structural elements, pumps, channels, and gated pores. In turn, these various functional units are comprised of complex proteins that translate the energy generated by the cell, and stored in ATP, into the work required to regulate ion homeostasis, transport nutrients and pump out wastes.
Figure 16: Far from being simple, passive, devising walls, cell membranes are complex biological machines consisting of structural elements, pumps, channels, and gated pores. In turn, these various functional units are comprised of complex proteins that translate the energy generated by the cell, and stored in ATP, into the work required to regulate ion homeostasis, transport nutrients and pump out wastes.
In the worst case, the cell membranes disappear as the structures they were, and reappear as new structures; droplets of membrane material, brand new micro-cells, and so on.[70] And they shed structures that were embedded, or enclosed in them; thus the explanation for the debris fields seen in frozen-thawed tissues that are inadequately cryoprotected.
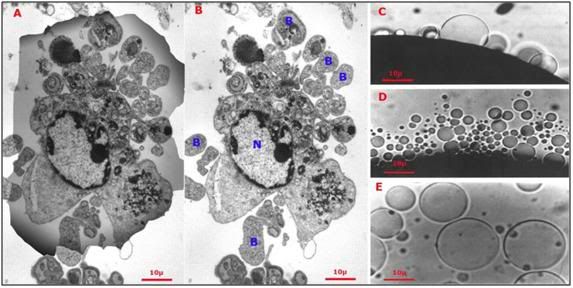 Figure 17: At left (A), the shadow of a hepatocyte is superimposed over the debris field (B) remaining after the cell was exposed to a hypertonic medium yielding approximately the same osmotic stress as would be experienced during freezing to -20oC (i.e., increase in incubating medium osmolality from ~310 mOsm to 3,720 mOsm). The cell has partially lyzed and has formed numerous large blebs (B) containing cytoplasmic material, as well as smaller plasma membrane vesicles devoid of interior structure. The intracellular organelles are largely unrecognizable with the exception of a few swollen mitochondria (M) and the cell nucleus (N). At right (C-D) hypertonicity-induced plasma membrane blebs and vesicles on the surface of a frog oocyte. The blebs and vesicles vary in size and were induced by exposure to a solution of approximately 3x normal tonicity (800 mOsm) – a fourth of the hyperosmotic stress imposed as a result of freezing to -20oC.[71]
Figure 17: At left (A), the shadow of a hepatocyte is superimposed over the debris field (B) remaining after the cell was exposed to a hypertonic medium yielding approximately the same osmotic stress as would be experienced during freezing to -20oC (i.e., increase in incubating medium osmolality from ~310 mOsm to 3,720 mOsm). The cell has partially lyzed and has formed numerous large blebs (B) containing cytoplasmic material, as well as smaller plasma membrane vesicles devoid of interior structure. The intracellular organelles are largely unrecognizable with the exception of a few swollen mitochondria (M) and the cell nucleus (N). At right (C-D) hypertonicity-induced plasma membrane blebs and vesicles on the surface of a frog oocyte. The blebs and vesicles vary in size and were induced by exposure to a solution of approximately 3x normal tonicity (800 mOsm) – a fourth of the hyperosmotic stress imposed as a result of freezing to -20oC.[71]
Because biological systems are not solid state devices, if they are damaged badly enough, they do not remain as discrete, broken pieces waiting to have their pre-injury, functioning structure inferred from the debris. And it is important to understand that some of the “re-morphing” that goes on during the cryopreservation process occurs as a consequence of the enormous osmotic and mechanical stresses imposed by ice formation – not as consequence of thawing (see Figure 17, above). Further, as previously noted, there are significant changes to membrane structure, such crystallization of the lipids that occur solely as a result of cooling, and completely independent of freezing. To return to the mobile phone analogy, it would be as if the phone were made up of liquids and gels encased in soap bubble membranes, and the device was crushed. Crushing is thus a much better analogy to use when describing cryoinjury to biological systems, than is breaking or shattering.
Imagine a soap bubble with an exquisitely detailed picture embedded into its surface. A picture made up of millions of tiny pixels comprised of colored micro-particles. If you burst the bubble, some of the bubble wall material will return to a simpler, all-fluid state, and some of it may reform into new bubbles. But in any event, the picture is gone, and what’s more, it is not obvious that the image can be inferred from the puddle of particles in liquid, and the new bubbles that result. In fact, given our current understanding of physical law, inference of the image that was on the surface of the bubble before it burst is not possible.
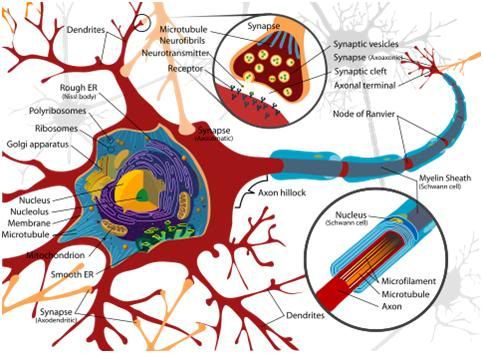
Figure 18: Simple schematic of a neuron and its axon, dendrites and synapses. The dendritic arbors that grow out of the neuronal soma and from the axon generate hundreds of thousands of synapses (up to 1 million per neuron) that serve as the signal switching mechanisms allowing inter-cellular communication and encoding LTM.
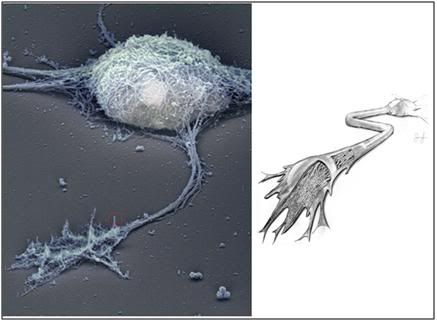 Figure 19: At left above, the cytoskeleton of a typical cerebral cortex neuron from the hippocampus. A representative dendrite has been circled in red and is shown in greater detail in Figure 20, below. This image was created using Rotary shadow electron microscopy of a cultured, wild-type, hippocampal neuron. The plasma membrane has been removed, allowing a detailed view of the underlying cytoskeleton. At right (above) is an artist’s cutaway rendering of the axon, showing the cytoskeleton, and microtubules that carry skeletal proteins, and other nutrients from the cell soma, to the dendrites and synapses. [Stern, S, Debre, E, Stritt, C, Berger, J, Posern, G, Knöll, B. A nuclear actin function regulates neuronal motility by serum response factor-dependent gene transcription. The Journal of Neuroscience, April 8, 2009, 29(14):4512-451: http://www.jneurosci.org/cgi/content/short/29/14/4512]
Figure 19: At left above, the cytoskeleton of a typical cerebral cortex neuron from the hippocampus. A representative dendrite has been circled in red and is shown in greater detail in Figure 20, below. This image was created using Rotary shadow electron microscopy of a cultured, wild-type, hippocampal neuron. The plasma membrane has been removed, allowing a detailed view of the underlying cytoskeleton. At right (above) is an artist’s cutaway rendering of the axon, showing the cytoskeleton, and microtubules that carry skeletal proteins, and other nutrients from the cell soma, to the dendrites and synapses. [Stern, S, Debre, E, Stritt, C, Berger, J, Posern, G, Knöll, B. A nuclear actin function regulates neuronal motility by serum response factor-dependent gene transcription. The Journal of Neuroscience, April 8, 2009, 29(14):4512-451: http://www.jneurosci.org/cgi/content/short/29/14/4512]
A typical cerebral cortex neuron in a mammal may have as many as one million synaptic interconnections with its neighboring neurons, yet each one is distinct, and will have different configurations of membrane proteins and structure at any instant in time. These synapses can cover the entire body of the neuron, including the dendrites, where they are observed as “synaptic spines,” using various kinds of electron microscopy. Electron microscopic examination with 3-dimensional reconstruction of a microscopic block of brain tissue demonstrates that these synapses are present at very high density in relation to each other.[72],[73],[74] In Figure 20 the incredible density and exquisitely complex synaptic connections of three dendritic spines branching from three different axons is shown. Each point of color (excluding green) is a functional point of synaptic contact. The topology and apposition of these structures must be conserved during cryopreservation in order to preserve LTM.
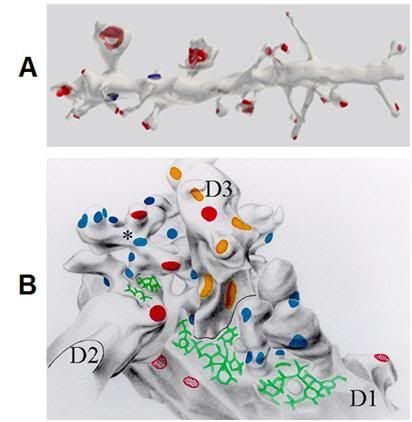 Figure 20: A: A 10 µ segment of pyramidal cell dendrite from stratum radiatum (CA1) with thin, stubby, and mushroom-shaped spines. Spine synapses colored in red, stem (or shaft) synapses colored in blue. The dendrite was made transparent in the lower image to enable visualization of all synapses.[75] B: Graphic reconstruction (i.e. manually shaded serial contour tracings) of dendritic spines arising from three dendrites (D1, D2, D3) participating in synaptic glomerule in thalamic ventrobasal nucleus. Some of spines are branched (asterisk). Multiple macular synapses of different afferent origin are marked in blue, red and orange. Extensive reticular adherent zones free of synaptic vesicles are marked in green. (Rat, thalamic ventrobasal nucleus.) (Adapted from Spacek and Lieberman 1974)[76]
Figure 20: A: A 10 µ segment of pyramidal cell dendrite from stratum radiatum (CA1) with thin, stubby, and mushroom-shaped spines. Spine synapses colored in red, stem (or shaft) synapses colored in blue. The dendrite was made transparent in the lower image to enable visualization of all synapses.[75] B: Graphic reconstruction (i.e. manually shaded serial contour tracings) of dendritic spines arising from three dendrites (D1, D2, D3) participating in synaptic glomerule in thalamic ventrobasal nucleus. Some of spines are branched (asterisk). Multiple macular synapses of different afferent origin are marked in blue, red and orange. Extensive reticular adherent zones free of synaptic vesicles are marked in green. (Rat, thalamic ventrobasal nucleus.) (Adapted from Spacek and Lieberman 1974)[76]
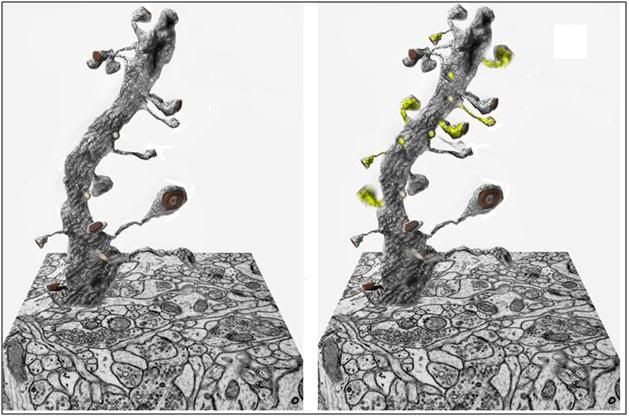 Figure 21: At left above is a three dimensional block of brain hippocampal tissue tomographically reconstructed from hundreds of slices of tissue 0.25 micron thick, using the same mathematical algorithms used in computerized tomography (CT) scanning employed in medical imaging. In the image above, a single dendrite has been isolated from the tissue block to show the number and configuration of its synapses. There are approximately 100 billion neurons in a typical 1.4 kg human brain, with an aggregate average of 0.15 quadrillion synapses.[77, 78] At right is a representation of a neuron having undergone synaptic remodeling in response to the encoding of long-term memory; with new synaptic connections highlighted in yellow.
Figure 21: At left above is a three dimensional block of brain hippocampal tissue tomographically reconstructed from hundreds of slices of tissue 0.25 micron thick, using the same mathematical algorithms used in computerized tomography (CT) scanning employed in medical imaging. In the image above, a single dendrite has been isolated from the tissue block to show the number and configuration of its synapses. There are approximately 100 billion neurons in a typical 1.4 kg human brain, with an aggregate average of 0.15 quadrillion synapses.[77, 78] At right is a representation of a neuron having undergone synaptic remodeling in response to the encoding of long-term memory; with new synaptic connections highlighted in yellow.
It is critically important, especially for the engineers, information technology, and computer scientists who are reading this to understand that the brain is not a computer, but rather, it is a massive, 3-dimensional hard-wired circuit. It does not use programming, addresses, or coding; and in engineering terms it most closely resembles pre-digital computer integrated circuits. Such circuits were constructed and wired for a discrete purpose and they did not function as multiple use processors. Similarly, the neuronal circuitry in the brain shows no evidence of using the biophysical equivalent of “addresses,” such as are used in digital computers. Each neuron has a single output axon which cannot select a receiver. Before addresses can work, they have to be agreed upon between the sender and the receiver, and such a function is problematic to have been generated as a result of biological evolution via natural selection.[7]
And of course, without addresses, there can be no ordered set of bits that carries information; and consequently no bytes, and no record. Coding, as is used in computers, is thus not possible: no location can be given to send or retrieve information from, and information cannot be moved from neuron to neuron. Another consequence of the inability of the brain to process information using programming, addresses and coding, is that information cannot be put side by side and compared. This means that information must be encoded and retrieved at the same location. A consequence of these insights is that, from an information processing standpoint, the brain is most properly viewed as a massive 3-dimensional homogenous memory array, with each memory location having its own processor, and every node and connection a site-dependent, dedicated purpose. Acute losses of circuit elements (neurons) are thus of far greater significance than are the loss of processors in a digital computer because they represent loss of the memory information they contain.
Molecular and Ultrastructural Basis of Long Term Memory
Of concern in the setting of cryonics is the nature of long term memory (LTM) and its likely response to the biophysical changes that may be imposed as a result of freezing and vitrification under different conditions. Several mechanisms are currently understood to be in play in the formation of LTM. The earliest of these is long-term potentiation (LTP), which is an enduring strengthening of signal transmission between two neurons that results from stimulating them synchronously.[79] LTP is but one of many mechanisms that facilitate the ability of chemical synapses in the brain to change their strength, and thus exhibit what is known as synaptic plasticity.[80] LTP appears to be the first step by which memories are encoded in the brain and it operates by modification of synaptic strength in response to artificial stimulation (simulated inter-neuronal signaling) via the delivery of carefully modulated electrical pulses, or naturally, as a result of multi-pass filtered and integrated somatosensory signaling, generated as a result of life experience.[81],[82],[83]
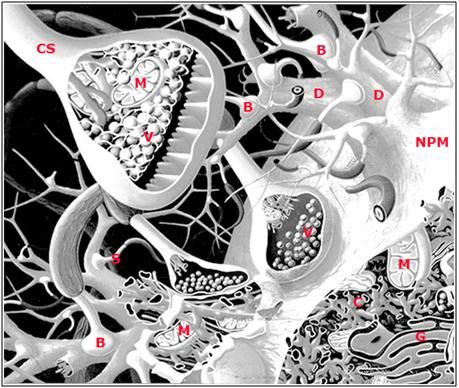 Figure 22: Artist’s rendering of some of the structural elements underlying long term potentiation (LTP): neuronal plasma membrane (NMP), dendrite (D), bouton (B), mitochondria (M), chemical synapse (CS), neurotransmitter vesicles (V), dendritic spine (S), cytoskeleton (C) and golgi apparatus (G). ). A necessary ‘defect’ to this rendering is that it shows far more extracellular space and much lower density of synapses than are actually present in the mammalian brain. A single axonal arbor is capable of sprouting tens of thousands of synapses.
Figure 22: Artist’s rendering of some of the structural elements underlying long term potentiation (LTP): neuronal plasma membrane (NMP), dendrite (D), bouton (B), mitochondria (M), chemical synapse (CS), neurotransmitter vesicles (V), dendritic spine (S), cytoskeleton (C) and golgi apparatus (G). ). A necessary ‘defect’ to this rendering is that it shows far more extracellular space and much lower density of synapses than are actually present in the mammalian brain. A single axonal arbor is capable of sprouting tens of thousands of synapses.
LTP has been the focus of a decade’s long investigation because it has in common with LTM rapid induction in response to experience or artificial stimulation, rapid induction of the synthesis of new proteins, the property of being associative in nature, and both LTP and LTM can last for many months in vivo in the free ranging animal.[79],[84] It has been posited that LTP may be the mechanism for encoding both broad classes of learning and memory: ranging from the most basic conditioned-responses present in unicellular organisms, to procedural learning, such as mastering a musical instrument, or learning how to drive a car, through the high-level cognitive memory involved in intellectual tasks such as understanding a scientific theory, comprehending complex social relationships, and recognizing individual faces and facial expressions.[79]
At the level of individual neurons, LTP enhances synaptic transmission by lowering the threshold for two neurons, one presynaptic and the other postsynaptic, to communicate with one another across a synapse. The precise molecular mechanics of how this alteration in synaptic sensitivity to signaling occurs are still not fully understood. Under laboratory conditions (usually employing brain slices in vitro) LTP occurs primarily as a result of alterations to the biochemistry and structure of the postsynaptic cell’s sensitivity to signals received from the presynaptic cell.[82] These signals occur secondary to the electrochemical stimulation of the presynaptic neuron, which results in the release of neurotransmitter chemicals (i.e., dopamine, serotonin, norephenephrine, etc.,) which activate neurotransmitter receptors present on the surface of the postsynaptic cell.
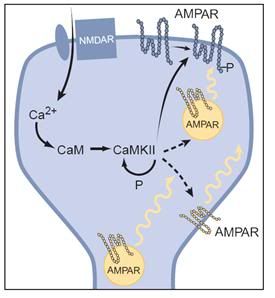 Figure: 23: Simplified schematic of the expression of LTP: An increase in calcium within the dendritic spine binds to calmodulin (CaM) to activate CaM Kinase II, which undergoes autophosphorylation, thus maintaining its activity after calcium returns to basal levels. CaMKII phosphorylates AMPA receptors (AMPARs) already present in the synaptic plasma membrane, thus increasing their single-channel conductance. CaMKII is also postulated to influence the sub-synaptic localization of AMPA receptors, such that more AMPA receptors are delivered to the synaptic plasma membrane. The localization of these “reserve” AMPA receptors is unclear, and thus they are shown in three different possible locations. Before the triggering of LTP, some synapses may be functionally silent in that they contain no AMPA receptors in the synaptic plasma membrane. Nevertheless, the same expression mechanisms would apply.[85]
Figure: 23: Simplified schematic of the expression of LTP: An increase in calcium within the dendritic spine binds to calmodulin (CaM) to activate CaM Kinase II, which undergoes autophosphorylation, thus maintaining its activity after calcium returns to basal levels. CaMKII phosphorylates AMPA receptors (AMPARs) already present in the synaptic plasma membrane, thus increasing their single-channel conductance. CaMKII is also postulated to influence the sub-synaptic localization of AMPA receptors, such that more AMPA receptors are delivered to the synaptic plasma membrane. The localization of these “reserve” AMPA receptors is unclear, and thus they are shown in three different possible locations. Before the triggering of LTP, some synapses may be functionally silent in that they contain no AMPA receptors in the synaptic plasma membrane. Nevertheless, the same expression mechanisms would apply.[85]
This activation of postsynaptic receptors results in a complex biochemical cascade inside the postsynaptic neuron which results in an increase of the postsynaptic cell’s sensitivity to neurotransmitter, in large measure by increasing the activity of existing neurotransmitter receptors (Figure 23), and by increasing the number of receptors on the postsynaptic cell surface.[82] In the minutes, hours or days following the initial induction of LTP there is gene activation in the neuronal nucleus resulting in the synthesis and incorporation of new proteins into the neuronal membrane, the creation of new synapses,[84] alterations to the structure of the neuronal cell membranes, and changes in the number and/or distribution of neurotransmitter containing vesicles in the synapses. There is also remodeling of the pattern and of the character of synaptic connections between neurons. LTP may also involve the participation of the glial cells – supportive cells which surround neurons and which secrete neurotrophic factors responsible for maintaining neuronal health and viability.[86] There is also emerging evidence that changes in neuronal membrane lipid structure may be important to LTP; for instance it has recently been demonstrated that lipids can interact directly with glutamate transporters.[87]
The creation of indefinitely durable LTM is presumably an even more complex process, and involves not just the addition, subtraction, or modification of one type of synapse, but of many. In fact, there are at least 161different synapse morphologies, a few of which are shown in Figure 24 and 25, below.[18] These synapse morphologies and distributions undergo both transient and long-lasting changes during LTP and LTM.[88],[89]
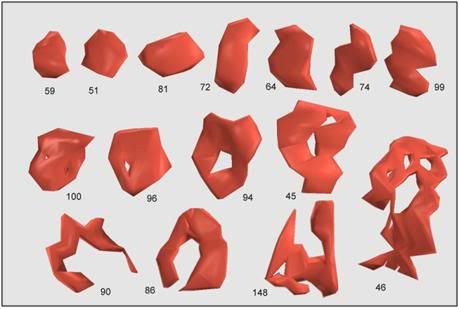 Figure 24: Most synapses cover a small area and have a compact, roughly convex shape, such as numbers 51, 59, and 81, above. These are referred to as macular synapses. Larger synapses are often exhibit ‘holes’ in the middle. These holes are regions of cell membrane devoid of the specializations characteristic of the synapse, e.g. postsynaptic density, synaptic cleft, presynaptic active zone, etc. Synapses with holes, such as numbers 45, 46, 86, 90, 94, 96, and 100, are referred to as perforated synapses. Of the 161 synapses so far classified in the neuropil, 148 are macular, while the remaining 13 are perforated. The difference between macular and perforated synapses can be seen in electron micrographs in which the postsynaptic densities have been stained (Figure 25).[89]
Figure 24: Most synapses cover a small area and have a compact, roughly convex shape, such as numbers 51, 59, and 81, above. These are referred to as macular synapses. Larger synapses are often exhibit ‘holes’ in the middle. These holes are regions of cell membrane devoid of the specializations characteristic of the synapse, e.g. postsynaptic density, synaptic cleft, presynaptic active zone, etc. Synapses with holes, such as numbers 45, 46, 86, 90, 94, 96, and 100, are referred to as perforated synapses. Of the 161 synapses so far classified in the neuropil, 148 are macular, while the remaining 13 are perforated. The difference between macular and perforated synapses can be seen in electron micrographs in which the postsynaptic densities have been stained (Figure 25).[89]
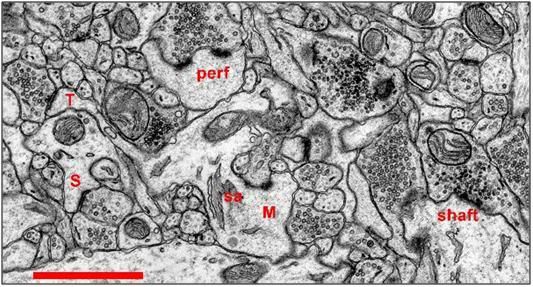 Figure 25: Synapses in Hippocampal Area CA1 of the Rat: Scale: 1 micron. Most synapses in stratum radiatum (>90%) occur on dendritic spines. As shown in Figure 3, spines come in a variety of shapes. A thin spine (T) has a small head with a macular postsynaptic density. The length of the spine neck is much greater than its diameter. The mushroom spine (M) has a large head, typically greater than 0.6 microns in diameter. An elaboration of the endoplasmic reticulum, called a spine apparatus (sa) is often visible within the neck of a mushroom spine. Mushroom spines also tend to have perforated (perf) postsynaptic densities on the spine head. The stubby spine (S) does not have a constricted neck, and its overall length is roughly equal to its diameter. The stubby spine illustrated above possesses a macular postsynaptic density. Occasionally synapses occur directly on the shaft of a dendrite (shaft) without the participation of a dendritic spine. Symmetric (inhibitory) synapses in stratum radiatum tend to be shaft synapses. All of the symmetric synapses of the neuropil shown in Figure 23 are shaft synapses. [Sorra KE, Harris KM (1993) Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J. Neurosci. 13:3736-3748. (5,414K PDF)]
Figure 25: Synapses in Hippocampal Area CA1 of the Rat: Scale: 1 micron. Most synapses in stratum radiatum (>90%) occur on dendritic spines. As shown in Figure 3, spines come in a variety of shapes. A thin spine (T) has a small head with a macular postsynaptic density. The length of the spine neck is much greater than its diameter. The mushroom spine (M) has a large head, typically greater than 0.6 microns in diameter. An elaboration of the endoplasmic reticulum, called a spine apparatus (sa) is often visible within the neck of a mushroom spine. Mushroom spines also tend to have perforated (perf) postsynaptic densities on the spine head. The stubby spine (S) does not have a constricted neck, and its overall length is roughly equal to its diameter. The stubby spine illustrated above possesses a macular postsynaptic density. Occasionally synapses occur directly on the shaft of a dendrite (shaft) without the participation of a dendritic spine. Symmetric (inhibitory) synapses in stratum radiatum tend to be shaft synapses. All of the symmetric synapses of the neuropil shown in Figure 23 are shaft synapses. [Sorra KE, Harris KM (1993) Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J. Neurosci. 13:3736-3748. (5,414K PDF)]
Another feature observed in LTP is that while each dendritic spine in the hippocampus typically receives only one excitatory synapse on its head, sometimes these synaptic heads are segmented into multiple active zones. These “segmented synapses” have evoked much speculation regarding their possible role in synaptic plasticity.[88] Recent reports show that segmented synapses increase transiently after LTP induction in the hippocampus, then return to control levels within an hour.[84],[90] The complex morphology and multi-axonal interface of a typical segmented synapse of the hippocampus is shown in Figure 26, below. While not common, if segmented synapses are indeed lasting, and material to LTM, then the number of effective connections in the brain will have to be revised substantially upwards.
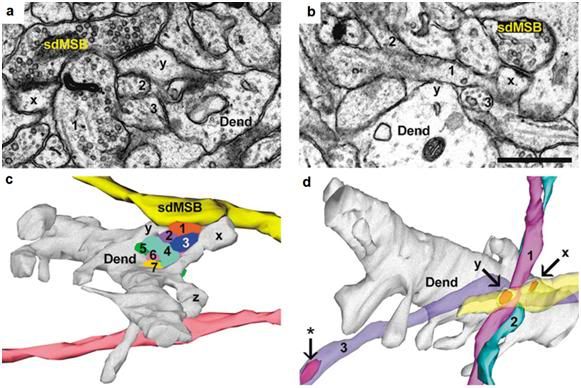 Figure 26: Reconstruction of ‘same-dendrite, multiple synapse boutons’ (sdMSBs) and related structures in a hippocampal brain slice. (a) The sdMSB makes a synapse with the head of one spine (x) on this section. Three of the axons (4,6,7) are visible between the spine head and the dendrite (Dend). (b) Three-dimensional reconstruction of the dendrite (gray), the sdMSB axon, and all seven axons (1–7) passing through the gap between the spines (x,y). Four of the axons (2,4,5,6) are cross-sectioned to avoid obscuring the other axons. Scale bar, 0.75 μm. [Fiala JC, Allwardt B, Harris KM. Dendritic spines do not split during hippocampal LTP or maturation. Nat Neurosci. 2002 Apr;5(4):297-8. PubMed PMID: 11896399.]
Figure 26: Reconstruction of ‘same-dendrite, multiple synapse boutons’ (sdMSBs) and related structures in a hippocampal brain slice. (a) The sdMSB makes a synapse with the head of one spine (x) on this section. Three of the axons (4,6,7) are visible between the spine head and the dendrite (Dend). (b) Three-dimensional reconstruction of the dendrite (gray), the sdMSB axon, and all seven axons (1–7) passing through the gap between the spines (x,y). Four of the axons (2,4,5,6) are cross-sectioned to avoid obscuring the other axons. Scale bar, 0.75 μm. [Fiala JC, Allwardt B, Harris KM. Dendritic spines do not split during hippocampal LTP or maturation. Nat Neurosci. 2002 Apr;5(4):297-8. PubMed PMID: 11896399.]
Understanding the Biophysical Consequences of Cryoinjury
As the foregoing discussion should make clear, the encoding of LTM likely relies upon a multiplicity of structural and biochemical changes, including the critical spatial relationship and apposition between dendrites, dendritic spines, neurons, and possibly even neuronal and glial cell membranes. It is within the context of this current understanding of the neurobiology of LTM that the effects of cryopreservation will now be considered.
Straight Freezing
Straight freezing is the cryopreservation of cells, tissues or organisms in the absence of added cryoprotection (in the form of colligative, or other cryophylactic molecules). Since most cells and organisms lack endogenous cryoprotection, the result is the conversion of almost all of the available water in the system into ice.
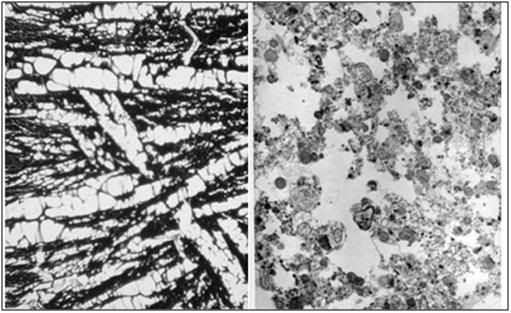 Figure 27: At left, above, is a light micrograph (400x) of the molecular layer[8] of the rabbit cerebral cortex subjected to freezing to -79oC in the absence of cryoprotection (straight freezing). The tissue is compressed between blocks of ice that have osmotically extracted the intracellular water. At right is the molecular layer of rabbit cerebral cortex tissue (10,000x) following thawing and fixation after straight freezing. The ultrastructure of the tissue resembles that of a tissue homogenate, rather than that of the molecular layer of the cerebral cortex.[91]
Figure 27: At left, above, is a light micrograph (400x) of the molecular layer[8] of the rabbit cerebral cortex subjected to freezing to -79oC in the absence of cryoprotection (straight freezing). The tissue is compressed between blocks of ice that have osmotically extracted the intracellular water. At right is the molecular layer of rabbit cerebral cortex tissue (10,000x) following thawing and fixation after straight freezing. The ultrastructure of the tissue resembles that of a tissue homogenate, rather than that of the molecular layer of the cerebral cortex.[91]
Undoubtedly the compressive forces are enormous under these conditions. What happens to the multiple species of brain cell membranes and their connections and embedded structures when they are dehydrated, biochemically destabilized, and then osmotically and mechanically compressed as a result of ice formation in straight freezing? Obviously, there will be shape changes in discrete membrane structures, but beyond that, to what extent will membranes merge, reorganize into novel structures, or otherwise become physically transformed in ways that would render inference of their pre-frozen state impossible – all of this occurring without thawing, and as a direct consequence of freezing? Definitive answers to these questions are not known, in part because the intense dehydration and compression of the tissue makes visualization of its ultrastructure virtually impossible in the frozen state.
In cryonics patients treated with straight freezing, these delicate and easily re-morphed structures will be crushed together and it might be impossible to tell, even with a complete 3-dimensional molecular level understanding of the remaining structure, what the original configuration was. It may be that receptors, membrane proteins, and other uniquely configured membrane structures, like the micro-particles comprising the hypothetical image on a soap bubble, will be scattered in debris fields and intermingled with each other.
The character of the changes observed in thawed-fixed, straight frozen brain tissue, as seen in Figure 27, above, suggest that irreversible structural degradation has occurred during the freezing process (and is undoubtedly amplified during thawing). The tissue shows no evidence of plasma cell membranes, and most intracellular structures are no longer identifiable; with the exception of the nucleus and mitochondria – both of which show major morphological abnormalities. Compare the TEM of the molecular layer of the straight frozen—thawed rabbit cerebral cortex at right in Figure 27, above, with that of the molecular layer of the cerebral cortex of a control animal in Figure 28, below. These data provide little grounds for optimism about the conservation of the fine structures of the brain believed to be responsible for encoding LTM.
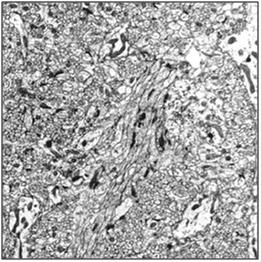 Figure 28: Control (perfusion fixed) TEM of the molecular layer of the rabbit cerebral cortex (10,000x ). [TEM by author]
Figure 28: Control (perfusion fixed) TEM of the molecular layer of the rabbit cerebral cortex (10,000x ). [TEM by author]
Low to Moderate Molarity Cryoprotected Freezing
To the extent that colligative cryoprotectant replaces water in the brain, the effects seen in Figure 27 are attenuated. In the case of freezing in the presence of ~ 4 M glycerol, the fine structure of the tissue before, during, and after freezing can be directly imaged. The sequence of events and the ultrastructural sequelae of cryopreservation under those conditions are shown in Figure 29, A-C, below.
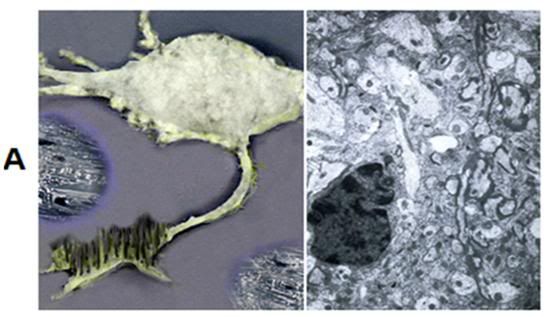 A: At left above is a rendering based upon the rotary shadow electron micrograph of a hippocampal neuron shown Figure 19. The neuron and the extracellular space have been equilibrated with 3.7 M glycerol, and freezing is beginning to take place as the neuron is progressively cooled to -79oC. The ice freezes out as pure water pushing an advancing front of hyperosmolar solution (purple) in front of it. At right is the condition of the tissue after glycerolization, but prior to the onset of freezing. [Artistic rendering and TEM by the author.]
A: At left above is a rendering based upon the rotary shadow electron micrograph of a hippocampal neuron shown Figure 19. The neuron and the extracellular space have been equilibrated with 3.7 M glycerol, and freezing is beginning to take place as the neuron is progressively cooled to -79oC. The ice freezes out as pure water pushing an advancing front of hyperosmolar solution (purple) in front of it. At right is the condition of the tissue after glycerolization, but prior to the onset of freezing. [Artistic rendering and TEM by the author.]
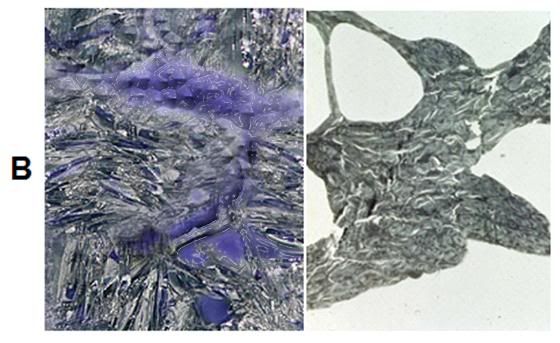 B: At top left is an artistic rendering of the neuron and the surrounding extracellular medium in the frozen state at -79oC. The neuronal cell body, as well as the axon and dendrites are dehydrated and compressed between masses of ice. The interior of the neuron, as well as small islands in the interstices between ice masses, contains highly concentrated, vitreous glycerol-water-salt solution (purple). At right(and below) is a TEM of rabbit hippocampus (21,000x ) that was frozen in the presence of 3.7 M glycerol and fixed in the frozen state with osmium tetroxide using a technique known as freeze substitution.[92] [PMC free article] The neuropil is compressed between masses of ice and several tears in the tissue are evident (red arrows). [Artistic rendering by the author; TEM courtesy of Brian Wowk, Ph.D.]
B: At top left is an artistic rendering of the neuron and the surrounding extracellular medium in the frozen state at -79oC. The neuronal cell body, as well as the axon and dendrites are dehydrated and compressed between masses of ice. The interior of the neuron, as well as small islands in the interstices between ice masses, contains highly concentrated, vitreous glycerol-water-salt solution (purple). At right(and below) is a TEM of rabbit hippocampus (21,000x ) that was frozen in the presence of 3.7 M glycerol and fixed in the frozen state with osmium tetroxide using a technique known as freeze substitution.[92] [PMC free article] The neuropil is compressed between masses of ice and several tears in the tissue are evident (red arrows). [Artistic rendering by the author; TEM courtesy of Brian Wowk, Ph.D.]
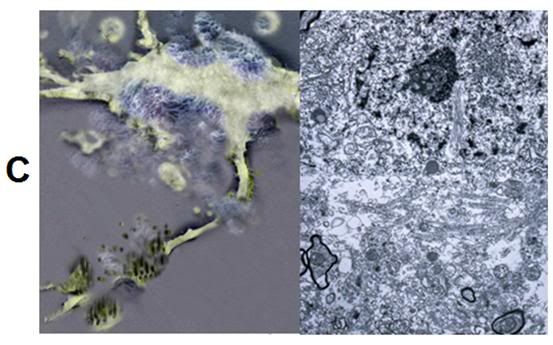 C: At left is an artistic rendering of the nature and extent of worst-case cryoinjury to a hippocampal neuron following freezing and thawing in the presence of 3.7 M glycerol. The axon has been transected by ice, the cell membrane has been osmotically stressed to the point of lysis, and there are debris surrounding the cell, in the form of membrane and cytoplasmic contents, as well as detached dendrites and synapses. Some of the plasma membrane has reformed into blebs and vesicles. At right is a TEM (9,000x) from the hippocampus of rabbit cerebral cortex that has been frozen to -79oC, rewarmed, perfused with fixative and prepared for TEM. The nucleus of a large neuron is visible in the center of the upper third of the micrograph, however the plasma membrane appears fragmented and there are ice-induced cavities with debris present in a band that spans the lower, middle third of the image. [Artistic rendering and TEM by the author.]
C: At left is an artistic rendering of the nature and extent of worst-case cryoinjury to a hippocampal neuron following freezing and thawing in the presence of 3.7 M glycerol. The axon has been transected by ice, the cell membrane has been osmotically stressed to the point of lysis, and there are debris surrounding the cell, in the form of membrane and cytoplasmic contents, as well as detached dendrites and synapses. Some of the plasma membrane has reformed into blebs and vesicles. At right is a TEM (9,000x) from the hippocampus of rabbit cerebral cortex that has been frozen to -79oC, rewarmed, perfused with fixative and prepared for TEM. The nucleus of a large neuron is visible in the center of the upper third of the micrograph, however the plasma membrane appears fragmented and there are ice-induced cavities with debris present in a band that spans the lower, middle third of the image. [Artistic rendering and TEM by the author.]
Figure 29: A-C, above: Impact of cryopreservation (-79oC) and thawing on tissue from the CA-1 area of the hippocampus in the presence of 4 M glycerol.
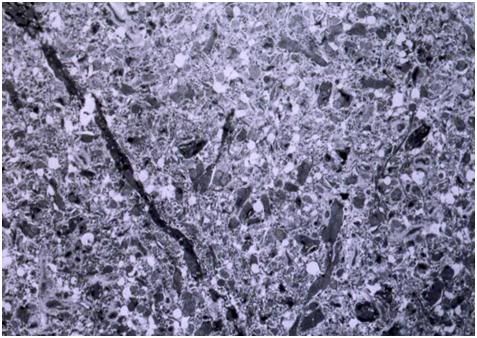
Figure 30: Appearance of feline cerebral cortex gray matter (medial temporal lobe) following freezing, thawing and fixation in the presence of 4 M glycerol (9000x). There is enhanced density of the ground substance (tissue texture) as a result of dehydration, and numerous small perforations of the neuropil that appear to be a result of ice formation. Overall, the fine structure appears well conserved and would seem to be inferable. [TEM by the author.]
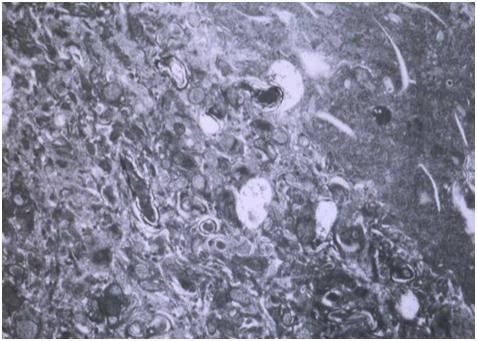
Figure 31: Appearance of feline cerebral cortex gray matter (medial temporal lobe) following freezing, thawing and fixation in the presence of 4 M glycerol (15,000x). There is enhanced density of the ground substance as a result of dehydration, but very little evidence of injury as a result of ice formation. Overall, the fine structure appears well conserved and would seem to be inferable. [TEM by the author.]
High Molarity Cryoprotection Freezing
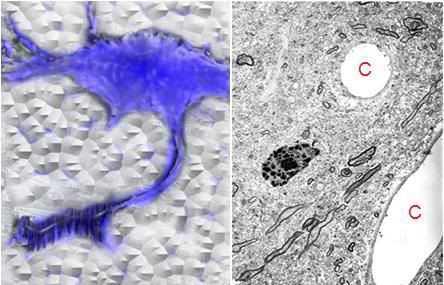
Figure 32: Artistic (left) rendering of a hippocampal neuron subjected to freezing to -79oC after equilibration with 7.5 M glycerol. Cell volume is conserved and ice formation is reduced to ~ 30% of the starting aqueous volume. At right is cerebral cortex tissue from the medial temporal lobe of a dog cryopreserved with 7.5 M glycerol, thawed, reperfused with fixative, and examined with transmission electron microscopy (15,000x). There is excellent conservation of the fine structure of the neuropil, and a notable absence of ice crystal artifacts, or debris. The capillaries (C) are intact and demonstrate uninterrupted adhesion of the endothelial cells to the basement membrane. The increased density of ground substance (tissue texture) and the presence of small spaces between ultrastructural elements are due to dehydration from the cryoprotectant agent (glycerol) and are reversible when the agent is removed. [Artistic rendering and TEM by the author.]
As the tissue cryoprotectant concentration approaches a vitrifiable amount (with concurrent suppression of ice formation) freezing damage is dramatically reduced. Not only is the volume of the system that is converted to ice greatly decreased, there is also a reduction in ice crystal size as result of the high cryoprotective agent (CPA) concentration. In the case of a CPA like glycerol, where the viscosity of the glycerol-water solution increases sharply, both as a function of increasing glycerol concentration and decreasing temperature, there is also a marked reduction in consolidation of smaller ice crystals into larger ones (recrystallization). The intracellular milieu becomes a glassy (vitreous) solid; in the case of systems cryoprotected with glycerol the glass transition point occurs at ~ -100oC.[93],
As can be seen in Figure 32 (above) and Figures 33 and 34 (below) the result of high molarity (glycerol) cryoprotection during freezing is dramatically improved conservation of tissue ultrastructure across anatomical locations and morphological levels. Synapses are intact with neurotransmitter vesicles exhibiting normal density and distribution. Cell membranes appear crisp and continuous, and there is no sign of blebbing or budding of vesicles from neuron, or other cell membranes.[91],[94]
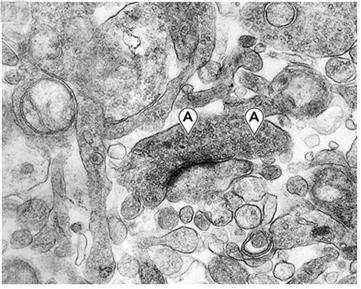 Figure 33: A synapse in gray matter from the hippocampus at 40,200x magnification. The presynaptic junction contains small packets of neurotransmitter (A) visible as granules. Note the overall crisp appearance of both the synaptic membranes and adjacent structures of the neuropil. This degree of preservation at the synaptic level was uniformly observed in all samples examined. [TEM by the author.[91]]
Figure 33: A synapse in gray matter from the hippocampus at 40,200x magnification. The presynaptic junction contains small packets of neurotransmitter (A) visible as granules. Note the overall crisp appearance of both the synaptic membranes and adjacent structures of the neuropil. This degree of preservation at the synaptic level was uniformly observed in all samples examined. [TEM by the author.[91]]
 Figure 34: White matter from the corpus collosum at 6700x magnification. Note the excellent preservation of the capillary (A) and its endothelial cell plasma membranes. The nucleus (B) shows typical loss or reorganization of nucleoplasm; this is seen more frequently in frozen-thawed brains than in brains just perfused with glycerol and fixed without freezing. Several axons (C) exhibit typical shrinkage of axoplasm and alteration in myelin structure. The increase in free space between axons and other structures is the result of glycerol-induced dehydration. [TEM by the author.[91]]
Figure 34: White matter from the corpus collosum at 6700x magnification. Note the excellent preservation of the capillary (A) and its endothelial cell plasma membranes. The nucleus (B) shows typical loss or reorganization of nucleoplasm; this is seen more frequently in frozen-thawed brains than in brains just perfused with glycerol and fixed without freezing. Several axons (C) exhibit typical shrinkage of axoplasm and alteration in myelin structure. The increase in free space between axons and other structures is the result of glycerol-induced dehydration. [TEM by the author.[91]]
Vitrification
Laboratory Experience
The only structural studies of vitrified mammalian brains which are known to exist are those conducted by, or under the auspices of 21st Century Medicine (21CM), a cryobiological research and development company located in Fontana, CA. The most definitive data so far disclosed employed M-22, a vitrification solution developed by 21CM, primarily for renal vitrification.[95]The composition of M-22 is shown Figure 35, below. This complex mixture of colligative and actively ice inhibiting cryoprotectants exhibits comparatively low toxicity, even at concentrations of ~60%. A unique feature of M-22 is the presence of two synthetic molecules that inhibit ice growth by binding to both the a and c axes of ice.[96],[97],[98] These molecules stabilize the solution against ice nucleation and propagation, allowing for use of the much slower cooling and rewarming rates needed for ice free cryopreservation of large tissues masses, such as humans organs.
 Figure 35: Twenty First Century Medicine’s M-22 vitrification solution contains 5 penetrating colligative cryoprotective agents as well as 6% of non-penetrating polymers – two of which are highly active ice-blocking molecules; Supercool X-1000 and Supercool Z-1000. X-1000 contains 80% of the syndiotactic stereochemical form of polyvinyl alcohol and 20% vinyl acetate and Z-1000 is a linear polymer of polyglcerol with an average molecular weight of 750 Da. Both bind to the a and c axes of ice crystals, stabilizing solutions they are present in against ice formation during slow rates of cooling and rewarming.[95][Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
Figure 35: Twenty First Century Medicine’s M-22 vitrification solution contains 5 penetrating colligative cryoprotective agents as well as 6% of non-penetrating polymers – two of which are highly active ice-blocking molecules; Supercool X-1000 and Supercool Z-1000. X-1000 contains 80% of the syndiotactic stereochemical form of polyvinyl alcohol and 20% vinyl acetate and Z-1000 is a linear polymer of polyglcerol with an average molecular weight of 750 Da. Both bind to the a and c axes of ice crystals, stabilizing solutions they are present in against ice formation during slow rates of cooling and rewarming.[95][Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
As a result of very recent advances in vitrification technology, it is now possible to vitrify entire mammalian organs.[99] However, two significant remaining problems are to inhibit ice growth during rewarming (“devitrification”) in some tissues that do not equilibrate well with the cryoprotectant chemicals, and to overcome the problem of fracturing, which occurs during cooling to or below Tg.
Ice formation occurs during rewarming as a result of the generation of ultramicroscopic ice nuclei during cooling, which cannot grow or propagate, because there is too little energy in the system and too little time for ice to grow as a result of steady and continued cooling to Tg. While devitrification is a significant hurdle to be overcome, it is a technological, rather than a theoretical one. Additionally, virtually all research on reversible vitrification of organs has been conducted on the kidney, and this presents a unique challenge, because the interior of the organ, the renal medulla, is very poorly circulated (Figure 36). It is thus difficult to load a sufficient concentration of cryoprotectants into this poorly vascularized tissue to completely avoid ice formation.[99]
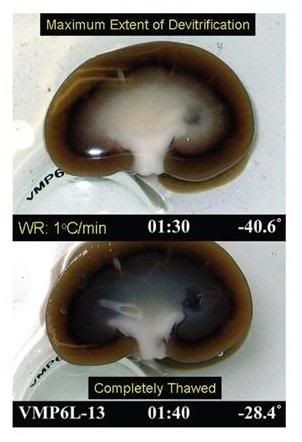 Figure 36: Visual appearance of ice in a rabbit kidney that was cross-sectioned during rewarming. The kidney was perfused with a cryoprotective mixture called M22 at -22°C, cut in half, immersed in M22, vitrified at -135°C, and eventually re-warmed at ~1°C/min while being periodically photographed. Times (1:30 and 1:40) represent times in hours and minutes fom the start of slow warming. The temperatures refer to ambient atmospheric temperatures near the kidney but not within the kidney itself. The upper panel shows the kidney at the point of maximum ice cross-sectional area, and the lower panel shows the kidney after complete ice melting. Both panels show the site of an inner medullary biopsy taken for differential scanning calorimetery in order to determine the actual concentration of cryoprotectants in the tissue with high precision. [http://cryoeuro.eu:8080/download/attachments/425990/FahyPhysicBiolAspectsRenalVitri2010.pdf?version=1&modificationDate=1285892563927]
Figure 36: Visual appearance of ice in a rabbit kidney that was cross-sectioned during rewarming. The kidney was perfused with a cryoprotective mixture called M22 at -22°C, cut in half, immersed in M22, vitrified at -135°C, and eventually re-warmed at ~1°C/min while being periodically photographed. Times (1:30 and 1:40) represent times in hours and minutes fom the start of slow warming. The temperatures refer to ambient atmospheric temperatures near the kidney but not within the kidney itself. The upper panel shows the kidney at the point of maximum ice cross-sectional area, and the lower panel shows the kidney after complete ice melting. Both panels show the site of an inner medullary biopsy taken for differential scanning calorimetery in order to determine the actual concentration of cryoprotectants in the tissue with high precision. [http://cryoeuro.eu:8080/download/attachments/425990/FahyPhysicBiolAspectsRenalVitri2010.pdf?version=1&modificationDate=1285892563927]
A fair summary of the current technological state of the art is that under ideal (laboratory) conditions, it is likely now possible to place complex mammalian organs, such as the rabbit kidney, into indefinitely long suspended animation with little or no loss of viability, and no damage as a consequence of structural disruption due to ice formation. The use of radio frequency, or microwave illumination to speed rewarming, the use of warm gas (such as helium) to perfuse the organ’s circulation, or a combination of these modalities, may offer a workable solution to the problem of ice formation during rewarming. Perhaps most impressively, one mammalian kidney has survived vitrification and rewarming sufficiently intact to permit immediate support of the rabbit from which it was removed (as the sole kidney), until the animal was sacrificed for evaluation 29 days after the organ was re-implanted.[99]
 Figure 37: The first kidney to survive vitrification shortly before it was removed from the animal for evaluation after supporting its life as the sole kidney for 29 days. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc, http://www.21cm.com/]
Figure 37: The first kidney to survive vitrification shortly before it was removed from the animal for evaluation after supporting its life as the sole kidney for 29 days. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc, http://www.21cm.com/]
An additional complicating factor in achieving reversible (viable) vitrification of the mammalian brain has been the inability to continue cryoprotectant perfusion at the same subzero temperatures (-20oC) that have proven essential for recovery of rabbit kidneys following loading and unloading with M-22. As can be seen in Figure 38, below, perfusion of the terminal concentration of M-22 is not possible below ~ -3-4oC. Exposure to ~8.2M M-22 at such a relatively high temperature, for the final ~60 min of perfusion required to load the brain with the CPA mixture results in major loss of viability, but does not visibly affect brain ultrastructure, as imaged using TEM.
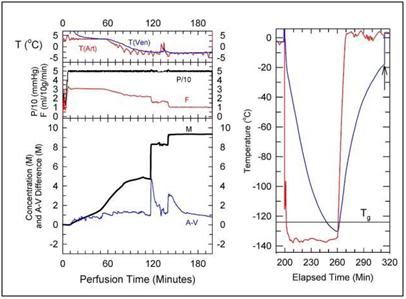 Figure 38: Cryoprotection and cooling protocol used to achieve structural vitrification of the rabbit brain at 21st Century Medicine, Inc., CPA loading commences at a temperature of ~+4oC and continues at that temperature for ~ 60 minutes while the M-22 concentration is gradually increased to ~4 M. The temperature is then reduced to ~ -3oC while the CPA concentration is increased to ~ 8M. The total time required to achieve full equilibration of the brain with M-22 is ~ 180 minutes, after which the organ is immediately transferred to an air-blast cooler for very rapid cooling to ~ -135oC. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
Figure 38: Cryoprotection and cooling protocol used to achieve structural vitrification of the rabbit brain at 21st Century Medicine, Inc., CPA loading commences at a temperature of ~+4oC and continues at that temperature for ~ 60 minutes while the M-22 concentration is gradually increased to ~4 M. The temperature is then reduced to ~ -3oC while the CPA concentration is increased to ~ 8M. The total time required to achieve full equilibration of the brain with M-22 is ~ 180 minutes, after which the organ is immediately transferred to an air-blast cooler for very rapid cooling to ~ -135oC. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
The ultrastructure of brains cryopreserved using the 21CM vitrification technique shows no evidence of ice formation. As previously noted, there is extensive dehydration of all imaged areas of the brain, with associated increase in ground substance density which makes assessment of very fine structures difficult. Reassuringly, it is possible to not only viably recover brain slices from this state, but to demonstrate the persistence of pre-vitrification induction of LTP in such vitrified and recovered brain slices.
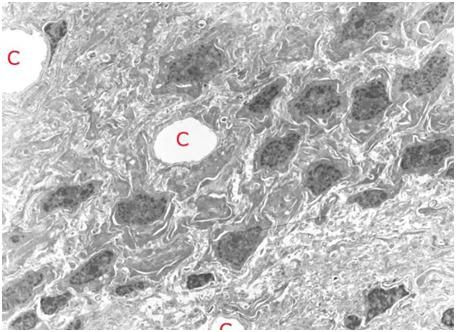 Figure 39: TEM of rabbit cerebral cortex gray matter (~ 15,000x) subjected to vitrification, rewarming and perfusion fixation using M-22 and the perfusion protocol shown in Figure 38, above. The extensive dehydration induced by cryoprotective loading makes it difficult to visualize the finer elements of the ultrastructure such as vesicles and microtubules. The overall appearance of tissue in terms of the larger structural elements and their relationship to each other is apparently normal. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
Figure 39: TEM of rabbit cerebral cortex gray matter (~ 15,000x) subjected to vitrification, rewarming and perfusion fixation using M-22 and the perfusion protocol shown in Figure 38, above. The extensive dehydration induced by cryoprotective loading makes it difficult to visualize the finer elements of the ultrastructure such as vesicles and microtubules. The overall appearance of tissue in terms of the larger structural elements and their relationship to each other is apparently normal. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
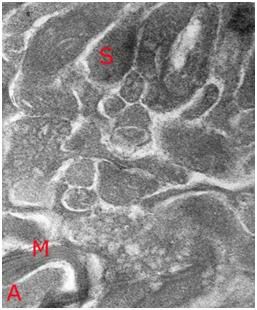 Figure 40: High magnification TEM (~ 40,000x) of vitrified rabbit brain tissue discloses the presence of difficult to visualize fine structures – in this case a synapse (S) with synaptic vesicles visible as dark densities in the synaptic bouton and a small myleinated (M) axon containing condensed axoplasm (A). Importantly, the topographical and structural relation of the synapse to the surrounding structures appears intact. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
Figure 40: High magnification TEM (~ 40,000x) of vitrified rabbit brain tissue discloses the presence of difficult to visualize fine structures – in this case a synapse (S) with synaptic vesicles visible as dark densities in the synaptic bouton and a small myleinated (M) axon containing condensed axoplasm (A). Importantly, the topographical and structural relation of the synapse to the surrounding structures appears intact. [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
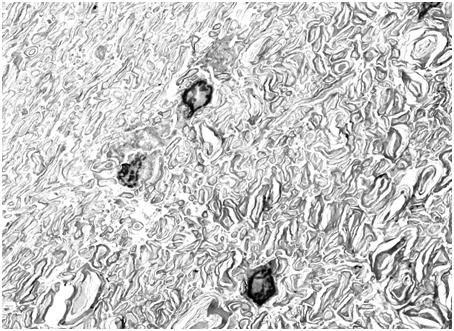 Figure 41: TEM of rabbit cerebral cortex white matter (15,000x) subjected to vitrification, rewarming and perfusion fixation using M-22 and the perfusion protocol shown in Figure 38, above. There is severe dehydration of the axoplasm and separation between some of the layers of myelin. There is no evidence of ice formation, and all structural changes appear to be a consequence of CPA-induced dehydration. These changes are reversible with controlled removal of CPA and return of the tissue to incubating medium (see Figure 42, below). [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
Figure 41: TEM of rabbit cerebral cortex white matter (15,000x) subjected to vitrification, rewarming and perfusion fixation using M-22 and the perfusion protocol shown in Figure 38, above. There is severe dehydration of the axoplasm and separation between some of the layers of myelin. There is no evidence of ice formation, and all structural changes appear to be a consequence of CPA-induced dehydration. These changes are reversible with controlled removal of CPA and return of the tissue to incubating medium (see Figure 42, below). [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
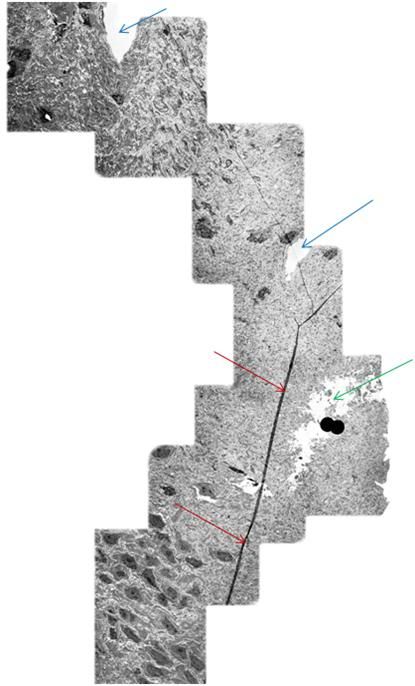 Figure 42: Mosaic of TEM’s demonstrating continuity of a long axon (red arrows) in a rabbit brain subjected to vitrification, rewarming and perfusion fixation using M-22 and the perfusion protocol shown in Figure 39, above. The tear in the tissue (green arrow) is believed to be a processing artifact. Two capillaries visible near the middle and top of the mosaic (blue arrows). [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
Figure 42: Mosaic of TEM’s demonstrating continuity of a long axon (red arrows) in a rabbit brain subjected to vitrification, rewarming and perfusion fixation using M-22 and the perfusion protocol shown in Figure 39, above. The tear in the tissue (green arrow) is believed to be a processing artifact. Two capillaries visible near the middle and top of the mosaic (blue arrows). [Image is courtesy of Brian Wowk, Ph.D., of 21st Century Medicine, Inc., http://www.21cm.com/]
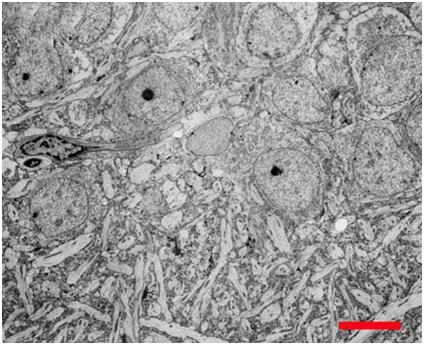 Figure 43: Hippocampal CA4 cells following recovery from vitrification using a fully reversible, viability conserving technique. Following rewarming and unloading of the CPA the tissue was incubated in artificial cerebrospinal fluid at 35oC for >60 min before being fixed in low-osmolality Karnovsky’s and examined by TEM.[100]
Figure 43: Hippocampal CA4 cells following recovery from vitrification using a fully reversible, viability conserving technique. Following rewarming and unloading of the CPA the tissue was incubated in artificial cerebrospinal fluid at 35oC for >60 min before being fixed in low-osmolality Karnovsky’s and examined by TEM.[100]
Both freezing and vitrification have the potential to disrupt the structures that encode LTM in ways that would leave them uninferrable. Vitrification may do this by the expedient of altering membrane structure irreversibly by dehydration, or by changing the molecular structure of the membranes (or membrane components) by directly perturbing their structure. Vitrification solution is not water, and water is critical to the structure of many of the molecules inside cells. Indeed, a good part of the science behind designing tolerable vitrification solutions is to make them behave as much like water as possible – while at the same time behaving as good, or good enough, glass forming agents when cooled.[49] On a purely structural basis it would seem that vitrification, applied under ideal (laboratory) conditions, is preserving the structures that encode memory and personality. To the extent that structural vitrification (as opposed to fully reversible, viable vitrification) perturbs or damages the biochemistry associated with LTP, there are grounds for concern. However, it seems unlikely that such injury would render the biochemistry of the brain uninferable, and therefore nonviable by the information-theoretic criterion for death.[22]
Real World Considerations
While laboratory investigations conducted under ideally controlled conditions provide considerable reassurance that existing cryopreservation techniques can conserve the essential structural and biochemical elements that comprise personal identity, such techniques are rarely available to human cryonics patients. Due to medico-legal and logistical constraints, most patients presenting for cryopreservation suffer extensive peri- and post-cardiac arrest global ischemia. When cryoprotection is delivered under these conditions in the laboratory setting, the results are very discouraging.
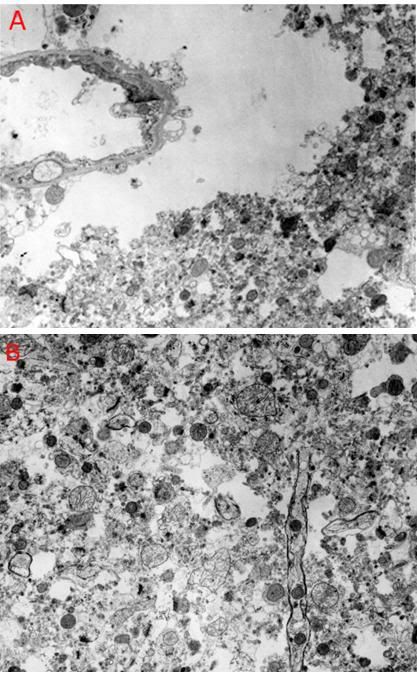 Figure 44: Feline cerebral cortex frozen, thawed and fixed in the presence of 4 M glycerol after 30 minutes of normothermic ischemia, followed by 24 hours of cold ischemia at ~2-4oC. There was severe disruption of the tissue fine structure by ice (A,B), in addition to changes associated with ischemia such as mitochondrial swelling and blebbing of the endothelial cells (A). [TEMs by the author.[101]
Figure 44: Feline cerebral cortex frozen, thawed and fixed in the presence of 4 M glycerol after 30 minutes of normothermic ischemia, followed by 24 hours of cold ischemia at ~2-4oC. There was severe disruption of the tissue fine structure by ice (A,B), in addition to changes associated with ischemia such as mitochondrial swelling and blebbing of the endothelial cells (A). [TEMs by the author.[101]
In 1983 studies were undertaken to determine the effects of 30 minutes of normothermic ischemia followed by 24 hours of cold ischemia at ~ 2-4oC.[101] Healthy adult cats were anesthetized; heparinized and cardiac arrest was induced. The animals were allowed to remain undisturbed on the operating table for 30 minutes, and then were packed completely in water ice, where they were allowed to remain for 24 hours. They were then perfused to 4 M glycerol using a linear increase in glycerol concentration during the ~ 60 minute perfusion interval. Following cryoprotective perfusion, the animals were cooled to dry ice temperature at ~ 3oC/hour, and to liquid nitrogen temperature at ~4oC/hour. Due to the unexpected presence of fracturing in the brain and other viscera, fixative reperfusion following thawing was not possible, and brain tissue samples for TEM were fixed by immersion.
As can be seen in Figure 44, above, there was extensive freezing damage superimposed over ischemic injury to the tissue. The mitochondria were swollen and often showed little internal structure. Neuronal plasma membranes were impossible to identify and the neuropil was macerated by what appeared to be ice artifacts. Large peri-capillary ice holes were almost uniformly present and large islands of tissue had the appearance of a tissue homogenate, as was observed in animals subjected to straight freezing.
The Problem of Ischemia and Ischemia-Reperfusion Injury
Hypoperfusion Following Reperfusion
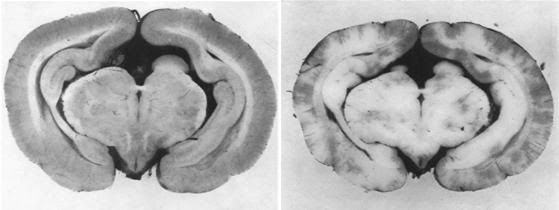 Figure 45: The no reflow phenomenon as documented by Ames, et al., in the rabbit brain in 1968. The control brain on the left was perfused with a colloidal carbon solution while the animal was still alive and under anesthesia. At right, the brain of an animal reperfused with carbon solution after 15 minutes of global normothermic cerebral ischemia – an interval of ischemia all too common in human cryopreservation patients. The pale white areas are zones of no-reflow or failed reperfusion, and account for 93% of the surface area in this cortical slice, despite use of hypertensive reperfusion at 120 mm Hg.[104]
Figure 45: The no reflow phenomenon as documented by Ames, et al., in the rabbit brain in 1968. The control brain on the left was perfused with a colloidal carbon solution while the animal was still alive and under anesthesia. At right, the brain of an animal reperfused with carbon solution after 15 minutes of global normothermic cerebral ischemia – an interval of ischemia all too common in human cryopreservation patients. The pale white areas are zones of no-reflow or failed reperfusion, and account for 93% of the surface area in this cortical slice, despite use of hypertensive reperfusion at 120 mm Hg.[104]
In cryonics patients, a significant contributor to reperfusion injury is hypoperfusion after the artificial restoration of circulation using closed chest cardiopulmonary support (CPS). Not only is CPS very inefficient at restoring adequate perfusion, blood flow to the brain, in particular, is impeded as a consequence of the cerebral “no-reflow” (CNR) phenomenon.[102] The no-reflow phenomenon was first described by Ames and Fisher in 1968,[103],[104] and was observed in the brains of animals subjected to reperfusion after experimentally induced global cerebral ischemia (GCI) from cardiac arrest lasting for periods of 5 minutes or longer. Interestingly, no-reflow does not usually develop in the heart, or other organs and tissues, until they have experienced periods of ≥ 30 min of normothermic global ischemia. Ames, et al., originally proposed three mechanisms as the cause of CNR; 1) glial and endothelial cell edema causing a reduction of the capillary lumen diameter to below the critical threshold for the passage of RBCs, 2) hyperviscosity of micro-vessel blood due to concentration of plasma proteins and formed blood elements as water is relocated from the microvascular space to intracellular space under the influence of the Gibbs-Donan Equilibrium in ischemia, and 3) alterations in the neurovascular endothelial surface as a result of bleb formation due to endothelial cell edema (See Figure 46).
 Figure 46: The no reflow phenomenon, first demonstrated by Ames and Fisher in 1968, constitutes a major barrier to successful cerebral resuscitation. Beginning at ~5 minutes after the start of normothermic global cerebral ischemia (GCI) a variety of biomechanical changes can be observed at the microscopic and especially at the ultramicroscopic level. Due to the failure of ion pumping cellular edema becomes pronounced at this point, resulting in swelling of both the brain parenchymal and capillary endothelial cells. This edema decreases capillary diameter to considerably less than that of the red blood cells (RBCs), leading to RBC plugging of the vessels. The cellular response to edema is to shed small vesicles of cell membrane material, apparently in an attempt to regulate cell volume and prevent hypervolemic cell lysis. These vesicles or “blebs” may interfere with the flow of the formed elements of the blood, and when they occur between the endothelium and the sarcolemma of the pre-capillary sphincters, they may also act to decrease capillary diameter resulting in reduced or absent blood flow.
Figure 46: The no reflow phenomenon, first demonstrated by Ames and Fisher in 1968, constitutes a major barrier to successful cerebral resuscitation. Beginning at ~5 minutes after the start of normothermic global cerebral ischemia (GCI) a variety of biomechanical changes can be observed at the microscopic and especially at the ultramicroscopic level. Due to the failure of ion pumping cellular edema becomes pronounced at this point, resulting in swelling of both the brain parenchymal and capillary endothelial cells. This edema decreases capillary diameter to considerably less than that of the red blood cells (RBCs), leading to RBC plugging of the vessels. The cellular response to edema is to shed small vesicles of cell membrane material, apparently in an attempt to regulate cell volume and prevent hypervolemic cell lysis. These vesicles or “blebs” may interfere with the flow of the formed elements of the blood, and when they occur between the endothelium and the sarcolemma of the pre-capillary sphincters, they may also act to decrease capillary diameter resulting in reduced or absent blood flow.
Additionally, there are rheological changes in the blood itself. As water translocates from the blood in the capillary lumen, plasma proteins and formed blood elements become concentrated and hyper-viscous, and consequently resistant to flow. The surfaces of the RBCs are prevented from sticking to each other due the existence of a charge barrier, the zeta potential, which is pH sensitive. Under conditions of acidosis, as pertain in ischemia, the zeta potential collapses and the RBCs become sticky and aggregate into masses which cannot pass through the micro-vessels. The RBCs themselves also undergo changes, most notably becoming rigid as a result of intracellular ATP depletion .This reduces RBC deformability, making their passage through brain capillaries difficult or impossible.
Finally, there is growing evidence that prolonged period of GCI cause activation of immune/inflammatory cascade resulting in neutrophil adhesion and activation, platelet aggregation and activation of the clotting cascade, the latter resulting in the possible formation of micro-thrombi in the small vessels, particularly in the brain venules.
Since the initial work by Ames, et al., it is has become apparent that the pathophysiology of CNR is considerably more complex. Other factors that have been identified as causative are changes in the character of the endothelial surface during ischemia, which make it stickier due to increases Nuclear factor kB (NFkB) and Tumor Necrosis Factor-α (TNF-α), resulting in elevated levels of a wide range of other pro-adhesion and pro-inflammatory chemokines.[105],[106] Additionally, the generation of free radicals, in particular peroxynitrite and the hydroxyl radical, degrade the extracellular matrix and the capillary basal lamina, resulting in increased permeability of the BBB with consequent development of interstitial cerebral edema.[107] Both free radical and leukocyte activity can also degrade the endothelial cell tight junctions to the point that frank extravasation of formed blood elements occurs, along with activation of the clotting cascade, resulting in the formation of fibrin lactoids and frank clots in the capillary lumen.[108] Free radical and leukocyte activation can also result in complete destruction of cells in the neurovascular endothelium, resulting in patches of exposed basement membrane, which in turn result in micro-thrombus formation. Blebs, debris from lyzed RBCs and endothelial cells, aggregated platelets, and precipitated macromolecules can also accumulate to form capillary occluding masses of debris (Figure 46).
The diameter of the average RBC is ~7.7μ, about 1μ larger than the diameter of the average brain capillary. In order for RBCs to pass through capillaries it is necessary for them to deform (and in so doing place the maximum amount of surface area in contact with the vascular endothelium to facilitate gas exchange). RBC deformability is critically dependent upon RBC intracellular adenosine triphosphate (ATP) concentration being adequate. With periods of ischemia of ~ 7 minutes, RBCs become depleted of ATP and become rigid, making passage through brain capillaries more difficult (higher arterial pressure required) or impossible.[109]

| Dispersed RBCs | Aggregated RBCs |
Figure 47: The zeta potential is the degree of negative charge on the surface of the RBC; the potential difference between the negative charges on the RBC and the cations in the fluid portion of the blood which constitutes of zone of charge the cells that prevents them from adhering to each other (above left). Changes in the cation content, or reduction of the pH of the plasma can collapse the zone of repulsive charge around the RBCs, allowing them to agglomerate and stick together in clumps.
The surface of RBC (and most cell) membranes is intrinsically sticky, and what keeps them from adhering to each other and aggregating into a solid mass is a complex interaction between sialic acid groups (sialoglycoprotein) on the RBC membrane, which give the cell a negative charge, and positive ions in plasma that are attracted to the negatively charged RBC membrane.[110] This zone of oppositely charged ions surrounding the RBC surface is called the “fixed layer.” Outside the fixed layer, there are varying compositions of ions of opposite polarities, forming a cloud-like zone. This area is called the “diffuse double layer,” and the whole of the diffuse double zone is electrically neutral. Thus, the net positive charge surrounding the RBCs keeps them apart due to electrostatic repulsion. The electrostatic potential of this zone, measured at the plane of hydrodynamic slippage outside the surface of the cell, is called the zeta potential.[111] Zeta potential is considered to be the electric potential of this inner area, including this conceptual “sliding surface” (Figure 48). As this electric potential approaches zero, particles tend to aggregate. Put more succinctly, the zeta potential is the degree of negative charge on the surface of the RBC, the potential difference between the negative charges on the RBC and the cations in the fluid portion of the blood.
 Figure 48: Graphic illustration of the zeta potential surrounding the RBC. The zeta potential consists of 3 layers of charge; the negative charge of the RBC membrane, a boundary layer of positively charged cations that travel with the cell as it moves through the blood plasma, and an outer layer of positive charge that is more dynamic composed of varying compositions of ions of opposite polarities, forming a cloud-like area that exists at the boundary of shear, or the “slipping phase” at the plane of hydrodynamic slippage outside the RBC. These last two layers are known as the “diffusible double layer.”
Figure 48: Graphic illustration of the zeta potential surrounding the RBC. The zeta potential consists of 3 layers of charge; the negative charge of the RBC membrane, a boundary layer of positively charged cations that travel with the cell as it moves through the blood plasma, and an outer layer of positive charge that is more dynamic composed of varying compositions of ions of opposite polarities, forming a cloud-like area that exists at the boundary of shear, or the “slipping phase” at the plane of hydrodynamic slippage outside the RBC. These last two layers are known as the “diffusible double layer.”
A critical determinant of the zeta potential is pH. The zeta potential of RBCs is optimized around a pH of 7.4. Under conditions of acidosis, and in particular where the pH drops below 6.6, as is the case in ischemia, the zeta potential of the RBC collapses and the intrinsic stickiness of the membrane surface is unmasked.[112] RBCs in contact with each other will tend to stick, and in the absence of flow (which acts as a dispersing agent) and under the influence of gravity, RBCs begin to aggregate and sediment out of the plasma.[113] Where RBCs are in contact with capillary endothelium, they will tend to stick, and the force required to dislodge them will be far larger than under physiological conditions. The aggregation of RBCs under acidic conditions was, at one time, used as an alternative method to centrifugation for removing RBCs from solution during washout of cryoprotectant following freezing, using a technique known as cytoagglomeration.[114]
Even in the absence of ischemia, dynamic variations in flow, pH and plasma chemistry can increase the amount of time RBCs in flowing blood spend in contact with each other. The longer the contact times between RBCs, the greater the viscosity of the blood. Increased blood viscosity, and the presence of loosely bound clumps or aggregates of RBCs, result in a blood sludging; a condition of greatly slowed and irregular flow in the micro-vessels (arterioles, capillaries and venules).[115]
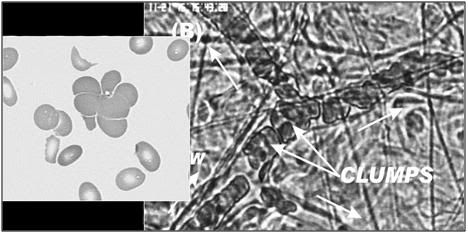 Figure 49: Aggregation or agglomeration of RBCs into irregular clumps is the most common pattern of RBC adhesion seen in both RCIRI and GCIRI. The impact on flow is devastating resulting in either severe blood sludging or complete arrest of microcirculatory flow.
Figure 49: Aggregation or agglomeration of RBCs into irregular clumps is the most common pattern of RBC adhesion seen in both RCIRI and GCIRI. The impact on flow is devastating resulting in either severe blood sludging or complete arrest of microcirculatory flow.
Within 60 seconds of the start of ischemia, pH in the cerebral micro-vessels quickly drops to ~6.8, and then declines to ~6.2 after only 5 minutes or more of GCI.[116]Sedimentation of RBCs, WBCs and platelets becomes pronounced after 6 minutes and is difficult to reverse due to the adhesion of the cells to each other.[117] The acidemia of prolonged ischemia may also adversely affect the configuration of some plasma proteins causing them to act as bridging molecules between the RBCs thus further increasing agglomeration.
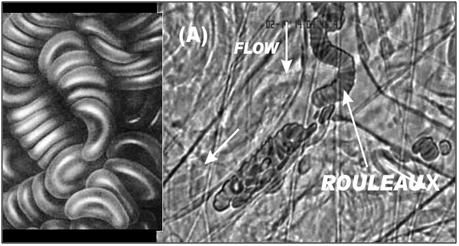 Figure 50: The adhesion of RBCs to each other in a “stack of coins” configuration, known as rouleaux is the less prevalent, but still common pattern of RBC aggregation observed during and after both RCIRI and GCIRI. As is the case with irregular aggregation of RBCs rouleaux formation has a profound negative impact on perfusion.
Figure 50: The adhesion of RBCs to each other in a “stack of coins” configuration, known as rouleaux is the less prevalent, but still common pattern of RBC aggregation observed during and after both RCIRI and GCIRI. As is the case with irregular aggregation of RBCs rouleaux formation has a profound negative impact on perfusion.
When flow is re-established, higher pressures and higher flow rates will be needed to generate the shear required to re-suspend sedimented blood cells, and to disrupt agglomerations of cells in the microvasculature.[118] With the passage of enough time (~30 min), agglomeration and sedimentation extend to the large vessels, where much larger masses of adherent cells will form, fall under the influence of gravity, and consolidate. This macro-scale hyper-viscous sludge will be very difficult to disrupt, and can be expected to compromise flow for prolonged periods of time following the start of reperfusion.
The work of Hossman, et al.,[119] and Sterz, et al.,[120] has demonstrated the critical importance of providing adequate circulatory support following global cerebral ischemia. Loss of autonomic regulation, depressed myocardial function secondary to ischemic insult of the myocardium, and autonomic dysfunction all serve to depress MAP and cerebral perfusion following restoration of circulation. Both Hossman’s and Sterz’s work has demonstrated significant improvements in neurological outcome if circulation is immediately supported extracorporeally during reperfusion – an option which is not available to cryonics patients.
In-house research conducted by the author has demonstrated that the cerebral microcirculation remains profoundly compromised for 30-60 minutes following reperfusion, even when circulation is restored using cardiopulmonary bypass. Brain parencymal and endothelial cell swelling, as well as changes in the zeta potential of the red blood cells, may all be contributing to the extensive blood sludging and microvascular stasis observed after reperfusion following 10 minutes of global normothermic ischemia in the laboratory.
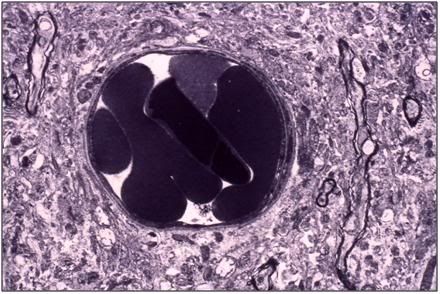 Figure 51: Canine cerebral cortical (gray matter) capillary plugged with a mass of red cells following extensive washing (5 L) with a colloid containing hyperosmotic solution, followed by fixation perfusion with Karnofsky’s fixative. [TEM by the Author.]
Figure 51: Canine cerebral cortical (gray matter) capillary plugged with a mass of red cells following extensive washing (5 L) with a colloid containing hyperosmotic solution, followed by fixation perfusion with Karnofsky’s fixative. [TEM by the Author.]
As the electron micrograph above demonstrates, even following attempts at reperfusion with a hyperosmotic physiological solution at a MAP of 90 mmHg, there are many instances of capillaries plugged with aggregated red cells, as seen above.
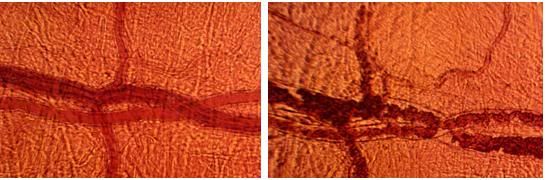 Figure 52: At left, baseline pial circulation in the dog brain prior to induction of cardiac arrest and the 15 minute ischemic insult. At right, flow is seen to be sluggish with obvious sludging in the larger vessels, and complete stasis observed in many of the smaller ones ~30 minutes after the onset of reperfusion. [Intravital microscopy by Jerry Leaf and the author.]
Figure 52: At left, baseline pial circulation in the dog brain prior to induction of cardiac arrest and the 15 minute ischemic insult. At right, flow is seen to be sluggish with obvious sludging in the larger vessels, and complete stasis observed in many of the smaller ones ~30 minutes after the onset of reperfusion. [Intravital microscopy by Jerry Leaf and the author.]
The microcirculation in the pia remains disturbed and sluggish even 30 minutes after reperfusion following 15 minutes of normothermic GCI as can be seen in Figure 51 which shows baseline flow in pial vessels and flow 30 minutes after ROSC at a MAP of 80 mmHg.
Consequences of Ischemia-Induced Derangement of the Microcirculation for the Human Cryopreservation Patient
In the case of human cryopreservation patients, it is apparent that the warm and cold ischemia suffered by many has effects on the brain (and somatic tissues) that combine features of GCIRI, RCIRI and Multisystem Organ Failure (MSOF, or the post-resuscitation syndrome). Injury associated with the post-resuscitation syndrome does not develop under normal clinical conditions until 12 to 24 hours after the ischemic insult. Leukocyte activation is “slow” (hours) and compromise to the BBB is typically delayed until 12 to 24 hours after reperfusion.[121]
 Figure 53: In the terminally ill patient suffering from progressive degenerative disease, the immune-inflammatory cascade is already activated. In cancer patients, NFkB and TNF-α will often be expressed and are responsible for the cachexia of the wasting syndrome. In some cancers (lung, prostate, colon), elements of the clotting cascade may be activated, and the patient will be in a hypercoagulable state weeks or months prior to cardiac arrest.
Figure 53: In the terminally ill patient suffering from progressive degenerative disease, the immune-inflammatory cascade is already activated. In cancer patients, NFkB and TNF-α will often be expressed and are responsible for the cachexia of the wasting syndrome. In some cancers (lung, prostate, colon), elements of the clotting cascade may be activated, and the patient will be in a hypercoagulable state weeks or months prior to cardiac arrest.
Activation of the Immune-Inflammatory Cascade
In the human cryopreservation patient a number of factors appear to be responsible for the acceleration of the inflammatory and tissue destroying components of reperfusion injury, causing them to manifest during the period of initial stabilization following cardiac arrest and the pronouncement of legal death (and especially by the time cryoprotective perfusion is initiated if there has been a prolonged period of cold ischemia; i.e., transport packed in ice absent perfusion and gas exchange). The first of these is the terminal illness, and any satellite pathologies that preceded, or were a consequence of it (Figure 53). As an example, a patient experiencing terminal decline from metastatic adenocarcinoma will inevitably experience up-regulation of a host of pro-inflammatory cytokines.[122],[123] With some cancers there will be chronic, low level activation of the clotting cascade as a direct result of the tumor’s biochemistry, and it should be noted that 50% of all cancer patients show evidence of deep vein thrombosis, or other kinds of intravascular clotting at autopsy.[124],[125]
Such patients will almost invariably experience ongoing localized ischemia as tumor compresses and compromises blood flow to healthy tissues; and there will also be ongoing necrosis in the center of the poorly vascularized tumor masses with attendant release of pro-inflammatory tissue breakdown products. Chronic and acute pain also up-regulate the immune-inflammatory cascade. Many medications used to treat the disease or its complications will adversely affect blood rheology leading to blood sludging and additional incomplete regional ischemia. Most neoplasms provoke enormous and sustained release of TNF-α which will have adverse effects on capillary integrity and blood coagulation. Finally, chemotherapy and radiation therapy both have dramatic pro-inflammatory systemic effects, as do the intercurrent infections that are so often complications of these treatments. Malnutrition with protein catabolism and cell death is yet another likely inflammation inducing factor.
 Figure 54: A prominent feature of moderate to severe ischemic injury in the absence of prompt blood washout and extracorporeal support in the cryonics patient is red trapping in the tissues. At left are photos taken of the arterial pump raceway and arterial filter at the conclusion of cryoprotective perfusion (4.5M glycerol). The circuit (L) of the patient given prompt CPS, followed by blood washout and continuous ECMO support until the start of CPA perfusion, shows only trace amounts of RBCs and has an unreadable hematocrit. The circuit shown (R) was from a patient who experienced sudden cardiac arrest, was given heparin and a brief interval of CPS in the hospital ED, and was then transported packed in ice with CPA perfusion starting ~20 hours later.
Figure 54: A prominent feature of moderate to severe ischemic injury in the absence of prompt blood washout and extracorporeal support in the cryonics patient is red trapping in the tissues. At left are photos taken of the arterial pump raceway and arterial filter at the conclusion of cryoprotective perfusion (4.5M glycerol). The circuit (L) of the patient given prompt CPS, followed by blood washout and continuous ECMO support until the start of CPA perfusion, shows only trace amounts of RBCs and has an unreadable hematocrit. The circuit shown (R) was from a patient who experienced sudden cardiac arrest, was given heparin and a brief interval of CPS in the hospital ED, and was then transported packed in ice with CPA perfusion starting ~20 hours later.
As a result of these destructive processes, the terminally ill patient is not only primed to experience the destructive effects of ischemia on the vascular endothelium, they have, in fact, often begun to experience many of the pathophysiological mechanisms of ischemia-reperfusion injury weeks, or even months before cardiac arrest occurs.
With the immune–inflammatory cascade already significantly activated, the peri-arrest period of severe regional ischemia (as tumor mass critically encroaches on vital organ structure and function) coupled with what are often very long (6-48 hours) peri-arrest intervals of severe hypoxic and ischemic injury during the agonal period, there is full-scale activation of the immune-inflammatory cascade. The result of this is that the patient is primed for reperfusion injury, and in fact may be experiencing reperfusion-type injury on both a regional and a systemic basis prior to cardiac arrest.
Pre-Cardiac Arrest Regional and Global Cerebral Ischemia?
Many patients dying slowly will experience the clinical signs of failed cerebral perfusion (fixed, unresponsive pupils, absence of corneal and deep tendon reflexes) anywhere from 30 minutes, to an hour or more prior to cardiac arrest. This typically occurs during a period of profound bradycardia (20-30 bpm) accompanied by 3-4 agonal breaths per minute, or erratic and infrequent agonal breaths (1-2 minute)[126] In some patients this condition may persist for many hours. In such cases there is clinical evidence at autopsy of the body (neuropatients) of systemic mixed regional and global ischemic injury, as indicated by the presence of clots in the femoral veins, pulmonary inflammation/edema, erosion of the gastric mucosa, and areas of focal necrosis in the liver, kidneys, and ileum, as determined by light microscopy.[127],[126] Rarely, onset of rigor has been observed in the gastrocnemius, peroneus longus and peroneus brevis muscles of the lower limbs an hour or more prior to cardiac arrest.[126]
Patients experiencing this kind of injury respond to CPA perfusion by developing severe peripheral and visceral edema, sometimes accompanied by transudation of large volumes of perfusate from the lungs (ET tube) and leakage of similarly large volumes of perfusate into the upper and lower gut, resulting in abdominal distension, and in some cases, compartment syndrome. Such patients invariably experience red cell trapping in the tissues which persists throughout cryoprotective perfusion.[128],[129],[130],[131],[132],[133] This is especially remarkable given the large volume of solution passed through the patient’s vasculature (120 L), the hyperosmolality of the CPA solution, and the relative cellular impermeability of a number of the cryoprotectants (i.e., glycerol, ethylene glycol). Patients with very short warm ischemic times (<8 minutes) who receive prompt and effective CPS, followed by blood washout and/or extracorporeal support, do not exhibit red cell trapping, and do not typically develop CPA perfusion limiting cerebral edema.[128],[129],[130],[131],[132],[133]
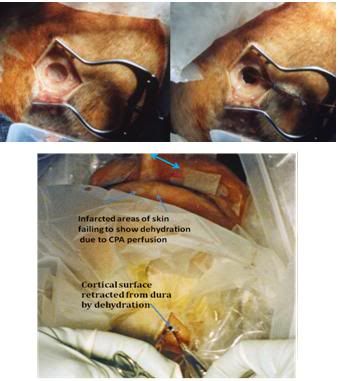
Figure 55: Patients with minimal peri-arrest ischemic injury who are given prompt and effective CPS followed by blood washout and either immediate CPA perfusion, or extended (~8-10 hours) ECMO support respond to CPA perfusion with massive cerebral dehydration (above right) and relatively uniform CPA perfusion of the skin. The patient shown below has a few infarcted areas evident on the bridge of the nose, probably secondary to pressure from ice bags, and is already evidencing cerebral cortical retraction from the dura, even during the first 30 minutes of CPA perfusion (7.5 M glycerol).
Cerebral Edema
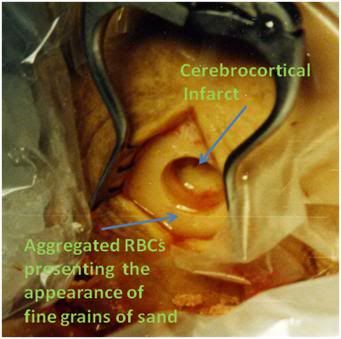 Figure 56: The burr hole of a human cryopreservation patient who experienced a brief period of CPS with heparinization, followed by packing in ice, and transport to the CPA perfusion facility. One hour into CPA perfusion, the brain abuts the burr hole opening, and a steady stream of aggregated RBCs can be seen exiting the burr hole in the perfusate leaking from the torn bridging veins between the dura and pia matters. The bridging veins tore as a consequence of the brief interval of cerebral dehydration which accompanied the initiation of CPA perfusion.
Figure 56: The burr hole of a human cryopreservation patient who experienced a brief period of CPS with heparinization, followed by packing in ice, and transport to the CPA perfusion facility. One hour into CPA perfusion, the brain abuts the burr hole opening, and a steady stream of aggregated RBCs can be seen exiting the burr hole in the perfusate leaking from the torn bridging veins between the dura and pia matters. The bridging veins tore as a consequence of the brief interval of cerebral dehydration which accompanied the initiation of CPA perfusion.
Compromise of the BBB, as indicated by the development of cerebral edema, may range from moderate to severe, and is often the reason for discontinuation of CPA perfusion.239, 240 In patients who are stabilized immediately post-arrest under good conditions, and who respond well to CPS (adequate EtCO2, SpO2, MAP) the BBB remains intact as evidenced by massive (~50%) shrinkage of the brain during CPA perfusion.[134],[135] Patients who have experienced high quality transport in the presence of minimal peri-arrest injury, but who are subsequently transported by air without continuous asanguineous cardiopulmonary bypass support, also suffer damage to the BBB, as evidenced by failure of brain to shrink (and/or remain shrunken) during CPA perfusion. In such patients there is often initial shrinkage of the brain in response to CPA loading, followed by return to isovolemia, or the development of a slight degree of cerebral edema by the end of CPA perfusion.[136],[137]
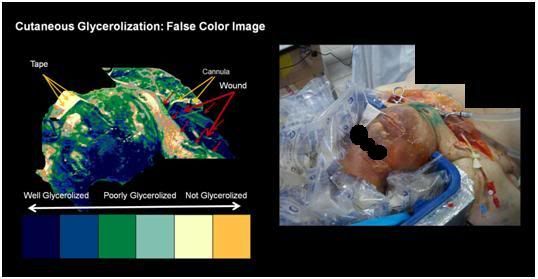 Figure 57: The impact circulatory obstruction on cryoprotectant equilibration in a cryonics patient who underwent ~24 hours of cold ischemia is evident in the inhomogeneous glycerolization of the skin at the conclusion of CPA perfusion. False color imaging discloses a patchwork of well, poorly, and completely unglycerolized areas of the patient’s skin. This pattern of patchy, compromised, or failed perfusion is present in the brain, as well as in the skin.
Figure 57: The impact circulatory obstruction on cryoprotectant equilibration in a cryonics patient who underwent ~24 hours of cold ischemia is evident in the inhomogeneous glycerolization of the skin at the conclusion of CPA perfusion. False color imaging discloses a patchwork of well, poorly, and completely unglycerolized areas of the patient’s skin. This pattern of patchy, compromised, or failed perfusion is present in the brain, as well as in the skin.
Cryonics patients who experience significant ischemic insult, be it peri- or post-cardiac arrest, warm or cold, will, as a consequence, suffer from multifocal areas of cerebral infarction and severely reduced flow on both the micro- and the macro-level. This will result in failed or inadequate cryoprotection of the brain, with many micro- and macro-domains of tissue undergoing straight freezing, or freezing in the absence of adequate cryoprotection. Patients who have suffered sufficiently long periods of warm and/or cold ischemia will be un-perfusable, and will of necessity be straight frozen. Given our current understanding of the biophysical basis of LTM, and in particular of “declarative” or “biographical” memory,” [138],[139],[140][9] it would appear that this critical element of personal identity is unlikely to survive cryopreservation under conditions of straight freezing, or in circumstances where the level of cryoprotection is very low, or very inhomogeneous, with substantial areas of the brain being subjected to neuronal membrane re-morphing, and severe topographical and spatial distortions of the neuropil as a result of ice formation and the attendant biochemical, osmotic, and mechanical stresses.
Summary
The prospects for the conservation of personal identity via cryopreservation under optimum conditions, particularly with the use of no, or very low ice forming methods, such as high molarity cryoprotective freezing or vitrification, seem excellent. TEM studies in animals demonstrate good preservation of both the gross and ultrafine structure of the neuropil, as well as of the white matter of the cerebral cortex. Synaptic connectivity and attachment to dendrites and dendritic spines appears undisturbed by these preservation methods, and where ice formation does cause injury, the structure appears to be displaced, rather than crushed or re-morphed – and as such, should allow inference of its undamaged, pre-cryopreservation state. The demonstration of the persistence of LTP following vitrification of mammalian hippocampal brain slices provides considerable grounds for optimism that the biochemical basis for encoding memory is also being conserved; at least in the case of vitrification under ideal conditions.
The criticality of avoiding both warm and cold ischemia cannot be overemphasized. The consequences of un-cryoprotected, or inadequately cryoprotected freezing are dire. There is nearly uniform loss of neuronal membrane structure, maceration of the neuropil, and obvious re-morphing of cell membrane components. These kinds of changes, especially if they are occurring during freezing, as well as during thawing, are not compatible with the survival of the patient using the information-theoretic criterion for determining death. Cryonicists may wish to rethink the way they communicate the viability of cryonics to the public – as well as how they conduct their personal lives – if they realistically hope to benefit from cryopreservation as a potentially reversible method of medical time travel.
References
1. Ben Best’s Cryonics FAQ [http://www.benbest.com/cryonics/CryoFAQ.html]
2. Frequently asked questions about cryonics. [http://www.alcor.org/FAQs/index.html, http://www.alcor.org/sciencefaq.htm]
3. Cryonics FAQ Part 1. [http://www.faqs.org/faqs/cryonics-faq/part1/]]
4. Jing Z, Sachs, F.: Alignment of tomographic projections using an incomplete set of fiducial markers. Ultramicroscopy 1991, 35( 2):37-43.
5. Soto G, Young, SJ, Martone, ME, et al.: Serial section electron tomography: a method for three-dimensional reconstruction of large structures. Neuroimage 1994, 1:230-243.
6. Stevens J, Davis, TL, Freidman, N, Sterling, P.: A systematic approach to reconstructing microcircuitry by electron microscopy of serial sections. Brain Res Rev 1980, 2:265-293.
7. Huijsmans D, Lamers, WH, Los, JA, et al.: Toward computerized morphometric facilities: a review of 58 software packages for computer-aided three-dimensional reconstruction, quantification, and picture generation from parallel serial sections. J Anat Rec 1986, 216(4):449-470.
8. Fiala J, Harris, KM.: Computer-based alignment and reconstruction of serial sections. Microscopy and Analysis 2002(January):5-7.
9. Fiala J, Harris, KM.: Extending unbiased stereology of brain ultrastructure to three-dimensional volumes. Journal of the American Medical Informatics Association 2001, 8(1):1-16.
10. Hixon H: Matching Grant Program for Fracture-Free Research & Development. In. Phoenix, AZ: Alcor Life extension Foundation; 2006-2007
11. Cryonics Institute Research [http://www.cryonics.org/research.html.]
12. Izquierdo I, Bevilaqua, LR, Rossato, JI, Bonini, JS, Medina, JH, Cammarota, M.: Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosc 2006, 29(9):496-505.
13. Abel T, Lattal, KM.: Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 2001, 11(2):180-187.
14. MacDonald J, Jackson, MF, Beazely, MA.: Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol 2006, 18(1-2):71-84.
15. Kim S, Linden, DJ.: Ubiquitous plasticity and memory storage. Neuron 2007, 56(4):582-592.
16. Blitzer R, Iyengar, R, Landau, EM.: Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry 2005, 57(2):113-119.
17. Lynch M: Long-term potentiation and memory. Physiol Rev 2004, 84(1):87-136.
18. Gruart A, Delgado-García, JM.: Activity-dependent changes of the hippocampal CA3-CA1 synapse during the acquisition of associative learning in conscious mice. Genes Brain Behav 2007 6(Suppl 1):24-31.
19. Costa-Mattioli M, Sonenberg, N.: Translational control of gene expression: a molecular switch for memory storage. Prog Brain Res 2008, 169:81-95.
20. Hawkins R, Kandel, ER, Bailey, CH.: Molecular Mechanisms of Memory Storage in Aplysia. Biological Bulletin 2006, 210:174-191.
21. LeDoux J: Synaptic Self: How Our Brains Become Who We Are. New York: Penguin Books; 2002.
22. Merkle R: The technical feasibility of cryonics. Med Hypotheses 1992, 39:6-16.
23. Dawkins R: The Blind Watchmaker. New York: Norton; 1988.
24. Bailey J, Pillard , RC.: A genetic study of male sexual orientation. Arch Gen Psychiatry 1991, 48:1089-1096.
25. Bailey J, Pillard, RC, Neale, MC. Agyei, Y.: Heritable factors influence sexual orientation in women. Arch Gen Psychiatry 1993, 50:217-223.
26. Hershberger S: A twin registry study of male and female sexual orientation. J of Sex Research 1997, 34:212-222.
27. Dunne M, Martin, NG, et al. : Genetic and Environmental influences on sexual orientation and its correlates in an Australian twin sample. J Pers Social Psychology 2000, 78:524-536.
28. Spalding K, Bhardwaj, RD, Buchholz, BA, Druid, H, et al.: Retrospective birth dating of cells in humans. J Cell 2005, 122(133-43).
29. Mazur P, Rall, WF, Rigopoulos, N.: Relative contributions of the fraction of unfrozen water and of salt concentration to the survival of slowly frozen human erythrocytes. Biophys J 1981, 36(3):653-675.
30. Mazur P: Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J General Physiol 1963, 47:347-369.
31. Meryman H: Tissue Freezing and Local Cold Injury. Physiol Rev 1957, 37(1):233-251.
32. Meryman H: Cryopreservation of living cells: principles and practice. Transfusion 2007, 47(5):935-945.
33. Abraham F: Homogeneous nucleation theory. New York: Academic Press.; 1974.
34. Lee R, Jr., Warren, GJ, Gusta, LV (Editors): Biological Ice Nucleation and Its Applications. St. Paul, Minnesota: APS PRESS; 1995.
35. Bank H, Brockbank, KG.: Basic principles of cryobiology. J Card Surg 1987(2(1 Suppl)):137-143.
36. Karlsson J, Toner, M.: Long-term storage of tissues by cryopreservation:critical issues. Biomaterials 1996, 17(3):243-256.
37. Mazur P: Freezing of living cells: mechanisms and implications. Am J Physiol 1984, 247((3 Pt 1):C):125-142.
38. Pegg D, Diaper, MP: Principles of cryopreservation. Methods Mol Biol 2007, 368:39-57.
39. Pegg D, Diaper, MP.: The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 2010, 60.((3 Suppl):S36-44).
40. Murthy S: Some insight into the physical basis of the cryoprotective action of dimethyl sulfoxide and ethylene glycol. Cryobiology 1998, 36(2):84-96.
41. Wowk B, Darwin, M, Harris, SB, Russell, SR, Rasch, CM.: Effects of solute methoxylation on glass-forming ability and stability of vitrification solutions. . Cryobiology 1999, 39(3):215-227.
42. Wowk B: Thermodynamic aspects of vitrification. Cryobiology 2010, 60(1):11-22
43. Fahy G: Vitrification: A new approach to organ cryopreservation. Prog Clin Biol Res 1986, 224:305-335.
44. Fahy GM, MacFarlane, D.R., Angell, CA., Meryman, HT.: Vitrification as an approach to cryopreservation. Cryobiology 1984, 21:407-426.
45. Tamiya T, Okahashi, N, Sakuma, R, Aoyama, T, Akahane, T, Matsumoto, JJ.: Freeze denaturation of enzymes and its prevention with additives. . Cryobiology 1985, 22(5):446-456.
46. Gordon-Kamm WS, PL.: Lamellar-to-hexagonal II phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci 1984, 81:6373- 6377.
47. Alberts ea: Molecular Biology of the Cell, 3rd Edition edn. New York: Garland Publishing; 1994.
48. Yeagle P: The Structure of biological membranes. Boca Raton: CRC Press; 2005.
49. Fahy G, Wowk, B, Wu, J, Paynter, S.: Improved vitrification solutions based on the predictability of vitrification solution toxicity. Cryobiology 2004, 48(1):22-35.
50. Fahy G. In: Cell Biology of Trauma. Edited by Oliver C, Lemasters, JJ Boca Raton: CRC Press; 1995: 333-356.
51. Balasubramanian S, Wolkers, WF, Bischof, JC.: Membrane hydration correlates to cellular biophysics during freezing in mammalian cells. Biochim Biophys Acta 2009, 1788(5):945-953.
52. Schwarz W: Temperature experiments on nerve and muscle membranes of frogs: Indications for a phase transition. Pflugers Arch 1979, 382(1):27-34.
53. Crowe J, Tablin, F, Tsvetkova, N, Oliver, AE, Walker, N, Crowe LM.: Are lipid phase transitions responsible for chilling damage in human platelets? Cryobiology 1999, 38:180-191.
54. Chow E, Chuang, SY, Tseng, PK.: Detection of a phase transition in red cell membranes using positronium as a probe. Biochim Biophys Acta 1981, 646(2):356-359.
55. Jacobson K, Papahadjopoulos, D.: Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry 1975, 14:152-161.
56. Fujikawa S: A freeze-fracture study designed to clarify the mechanisms of freezing injury due to the freezing-induced close apposition of membranes in cortical parenchyma cells of mulberry. Cryobiology 995, 32:444-454.
57. Guldbrand L, Jonsson, B., Wennerstrom, H.: Hydration forces and phase equilibria in the dipalmitoyl phosphatidylcholine-water system. J Coll Int Sci 1982, 89:532-541.
58. Wolfe J, et al.: Cellular cryobiology: thermodynamic and mechanical effects. International Journal of Refrigeration 2001, 24:438-450.
59. Fahy G: Cryoprotectant toxicity neutralization. Cryobiology 2010, 60 ((3Suppl):S45-53).
60. Khirabadi B, Fahy, GM, Saur, J, Ewing, L, Meryman, HT.: Failure of rabbit kidneys to survive chilling to -30′C after perfusion with 8M cryoprotectant at -3′C. Cryobiology 1994, 31:596-597.
61. Fahy G, Saur, J, Williarns, RJ.: Physical problems with vitrification of large systems. Cryobiology 1990, 27:492-510.
62. Khirabadi B, Fahy, GM, Ewing, L, Saur, J, Meryman, HT.: 100% survival of rabbit kidneys chilled to -32′C after perfusion with 8M cryoprotectant at -22′C. Cryobiology 1994, 31:597.
63. Fahy G, Saur, J, Williams, RJ.: Physical problems with the vitrification of large biological systems. Cryobiology 1990, 27(5):492-510.
64. Kroener C, Luyet, B.: Discontinuous change in expansion coefficient at the glass transition temperature in aqueous solutions of glycerol. Biodynamica 1966, 10:41-45.
65. Kroener C, Luyet, B.: Formation of cracks during the vitrification of glycerol solutions and disappearance of the cracks during rewarming. Biodynamica 1966, 10:47-52.
66. Rabin Y, Taylor, MJ, Wolmark, N.: Thermal expansion measurements of frozen biological tissues at cryogenic temperatures. J Biomechan Eng 1998, 120:259-266.
67. Rabin Y, Bell, E.: Thermal expansion measurements of cryoprotective agents. Part II: measurements of DP6 and VS55, and comparison with DMSO. Cryobiology 2003, 46::264-270.
68. Baicu S, Taylor, MJ, Chen, Z, Rabin, Y.: Vitrification of carotid artery segments: An integrated study of thermophysical events and functional recovery towards scale-up for clinical applications. Cell Preservation Technology 2006, 4(4):236-244.
69. Crompton M: The mitochondrial permeability transition pore and its role in cell death. Biochem J 1999, 341:233-249.
70. Pegg D, Diaper, MP.: On the mechanism of injury to slowly frozen erythrocytes. Biophys J 1988, 54(3):471-488.
71. Zhang Y, Gao, Feng, Popov, VL, Wen, W, Hamill, OP.: Mechanically gated channel activity in cytoskeleton deficient plasma membrane blebs and vesicles from Xenopus oocytes. Journal of Physiology 2000, 523(1):117-130.
72. Spacek J, Hartmann, M.: Three-dimensional analysis of dendritic spines. I. Quantitative observations related to dendritic spine and synaptic morphology in cerebral and cerebellar cortices. Anat Embryol 1983, 167:289-310.
73. Spacek J: Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anat Embryol 1985, 171:235-243.
74. Spacek J: Three-dimensional analysis of dendritic spines: Glial sheath. III. Anat Embryol 1985, 171:245-252.
75. Harris K, Stevens, JK.: Dendritic spines of CA1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci 1989, 9:2982-2997.
76. Spacek J L, AR.: Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. J Anat Rec (Lond) 1974, 117:487-515.
77. Pakkenberg B, Pelvig, D, Marner,L, Bundgaard, MJ., Gundersen, HJG., Nyengaard, JR, Regeur, L.: Aging and the human neocortex. Exp Gerontology 2003, 38:95-99.
78. Pakkenberg B, Gundersen, HJG.: Neocortical neuron number in humans: effect of sex and age. J Comp Neurology 1997, 384:312-320.
79. Cooke S, Bliss ,TV.: Plasticity in the human central nervous system. Brain Res Rev 2006, 129((Pt 7)):1659-1673.
80. Hölscher C: Synaptic plasticity and learning and memory: LTP and beyond. Neurosci Res 1999, 58(1):62-75.
81. Bliss T, Collingridge, GL.: A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993, 361(6407):31-39.
82. Malenka R, Bear, M.: LTP and LTD: an embarrassment of riches. Neuron 2004, 44(1):5-21.
83. Bear M: A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A 1996, 93(24):13453-13459.
84. Toni N, Buchs, PA, Nikonenko, I, Bron, CR, Muller, D.: LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 1999 402(6760):421-425.
85. Malenka R, Nicoll, RA.: Long-term potentiation–a decade of progress? Science 1999, 285(5435):1870-1874.
86. Achour SB, O, et al.: Glia: The many ways to modulate synaptic plasticity. Neurochemistry International 2010, 57(4):440-445.
87. Boudker O, Ryan, RM, Yernool, D, Shmimamot, K, Gouaux, E.: Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 2007, 445:387-393.
88. Jones D, Harris, RJ.: An analysis of contemporary morphological concepts of synaptic remodelling in the CNS: perforated synapses revisited. Rev Neurosci 1995, 6(177-219).
89. Sorra K, Harris, KM.: Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J Neurosci 1993, 13(5):3736-3748.
90. Toni Nea: Ca2+ Dependency of N-Cadherin Function Probed by Laser Tweezer and Atomic Force Microscopy. J Neurosci 2001, 21: 6245-6251.
91. Darwin M, Russell, S, Wakfer, P, Wood, L, Wood, C.: Effect of a human cryopreservation protocol on the ultrastructure of the canine brain. (Originally published by BioPreservation, Inc, as BPI Tech Brief 16 on CryoNet and SciCryonics, May 31, 1995), http://wwwalcororg/Library/html/braincryopreservation2html and http://wwwalcororg/Library/html/braincryopreservation1html.
92. van Harreveld A, Crowell, J, Malhotra, SK.: A study of extracellular space in central nervous tissue by freeze-substitution. J Cell Biol 1965, 25:117-137.
93. Li D, Liu, BL, Liu, YS, Chen, CL.: Predict the glass transition temperature of glycerol-water binary cryoprotectant by molecular dynamic simulation. Cryobiology 2008, 56(2):114-119.
94. Lemler J, Harris, SB, Platt, C, Huffman, T.: The arrest of biological time as a bridge to engineered negligible senescence. Ann NY Acad Sci 2004, 1019:559-563.
95. Wowk B, Fahy GM.: Toward large organ vitrification: extremely low critical cooling and warming rates of M22 vitrification solution. Cryobiology 2005, 51:362.
96. Wowk B, Leitl E, Rasch, CM, Mesbah-Karimi, N, Harris, SB, Fahy, GM.: Vitrification enhancement by synthetic ice blocking agents. Cryobiology 2000, 40(3):228-236.
97. Wowk B: Anomalous high activity of a subfraction of polyvinyl alcohol ice blocker. Cryobiology 2005, 50(3):325-331.
98. Wowk B, Fahy, GM.: Inhibition of bacterial ice nucleation by polyglycerol polymers. Cryobiology 2002, 44(1):14-23.
99. Fahy G, Wowk, B, Pagotan, R, et al.: Physical and biological aspects of renal vitrification. Organogenesis 2009, 5(3):167-175.
100. Pichugin Y, Fahy, GM, Morin, R.: Cryopreservation of rat hippocampal slices by vitrification. Cryobiology 2006, 52(2):228-240.
101. Darwin M, Leaf, JD.: Cryoprotective perfusion and freezing of the ischemic and nonischemic cat: http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1389, http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1390, http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1391, http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1392 See also: Federowicz, MG. and Leaf JD. Cryonics. issue 30, p.14,1983.
102. Fischer E, Ames, A III.: Studies on Mechanisms of Impairment of Cerebral Circulation Following Ischemia: Effect of Hemodilution and Perfusion Pressure. Stroke 1972, 3: 538.
103. Ames A, III, et al.: Cerebral ischemia II. The no-reflow phenomenon. Amer J Pathol 1968., 52:437-453.
104. Fischer E, Ames, A, III, Hedley-Whyte, ET, et al.: Reassessment of cerebral capillary changes in acute global ischemia and their relationship to the “no reflow phenomenon.”. Stroke 1977, 8:36-43.
105. Hallenbeck J: Cytokines, macrophages, and leukocytes in brain ischemia. Neurology 1997, 49:S5-S9.
106. Feuerstein G, Wang, X, Barone, FC.,. In: Ginsberg MD, Bogousslavsky J, eds.: Inflammatory mediators and brain injury: the role of cytokines and chemokines in stroke and CNS diseases. In: Cerebrovascular Diseases Cambridge, Mass: Blackwell Science; 1998: 507–531.
107. Avery SC, HA, Russell, RR.: Evolution and resolution of oedema following severe temporary cerebral ischaemia in the gerbil. J Neurol Neurosurg Psychiatry 1984, 47:604-610.
108. Tu Y, Heros, RC, Candia, G, Hyodo, A, Lagree, K, Callahan, R, Zervas, NT, Karacostas, D.: Isovolemic hemodilution in experimental focal cerebral ischemia. Part 1: Effects on hemodynamics, hemorheology, and intracranial pressure. . J Neurosurg 1988 69:72-81.
109. Weed R, LaCelle, PL, Merrill, EW.: Metabolic dependence of red cell deformability. J Clin Invest 1969, 48(5):795-809.
110. Oka S: Physical theory of some interface phenomena in hemorheology. Ann N Y Acad Sci 1983, 416:115-127.
111. Jan K, Chien, S.: Influence of the ionic composition of fluid medium on red cell aggregation. J Gen Physiol 1973, 61(5):655-668.
112. Jan K, Chie, S.: Role of surface electric charge in red blood cell interactions. Gen Physiol 1973, 61(5):638-654.
113. Baskurt O, Farley, RA, Meiselman, HJ.: Erythrocyte aggregation tendency and cellular properties in horse, human, and rat: a comparative study. Am J Physiol Heart Circ Physiol 1997, 273:H2604-H2612.
114. Tullis J, Lionetti, FJ.: Preservation of Blood by Freezing. Anesthesiology 1966, 27(4):483-493.
115. Chien S, Jan, KM.: Ultrastructural basis of the mechanism of rouleaux formation. Microvasc Res 1973, 5:155-166.
116. Lipowsky H, Kovalcheck, S, Zweifach, B.: The distribution of blood rheological parameters in microvasculature of cat mesentery. Circ Res 1978, 43:738-749.
117. Nemoto E, Frank, S.: Brain tissue pH after global brain ischemia and barbiturate loading in rats. Stroke 1981, 12:77-82.
118. Mchedlishvili G, Gobejishvili, L, Beritashvili, N.: Effect of intensified red blood cell aggregability on arterial pressure and mesenteric microcirculation. Microvasc Res 1993, 45:233-242.
119. Hossmann K: Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock 1997, 8(2):95-101; discussion p. 102-103.
120. Sterz F, et al.: Multifocal cerebral blood flow by Xe-CT and global cerebral metabolism after prolonged cardiac arrest in dogs. Reperfusion with open-chest CPR or cardiopulmonary bypass. Resuscitation 1992, 24(1):27-47.
121. Mossakowski M, Lossinsky, AS, Pluta, R, Wisniewski, HM.: Abnormalities of the blood-brain barrier in global cerebral ischemia in rats due to experimental cardiac arrest. . Acta Neurochir Suppl (Wien) 1994, 60:274-276.
122. Martinet N, Charles, T., Vaillant, P, et al.: Characterization of a tumor necrosis factor-alpha inhibitor activity in cancer patients. Am J Respir Cell Mol Biol 1992, 6:510-515.
123. Ueda T, Shimada, E, Urakawa, T.: Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol 1994, 29:423-429.
124. Green K, Silverstein, RL.: Hypercoagulability in cancer. Hematol Oncol Clin North Am 1996 10(2):499-530.
125. Rickles F, Levine, M, Edwards, RL.: Hemostatic alterations in cancer patients. Cancer and Metastasis Reviews, 11(3-4):237-248.
126. Darwin M: Unpublished case report of Alcor Life Extension Foundation patient Eugene Theodore Donovan, A-1169, 21 March, 1989. 1989.
127. Darwin M: Cryopreservation case report: Jerome Butler White. posted to CryoNet on 09 Jul 1994 03:02:55 EDT http://wwwcryonetorg/cgi-bin/dspcgi?msg=2868, see also: http://wwwcryonetorg/cgi-bin/dspcgi?msg=2867 and http://wwwcryonetorg/cgi-bin/dspcgi?msg=2874 1994.
128. Darwin M: Unpublished case report of Alcor Life Extension Foundation patient A-1133, August, 1987. 1987.
129. Leaf J, Federowicz, M, Hixon, H.: Case report: two consecutive suspensions, a comparative study in experimental human suspended animation. Cryonics 1985, 6(11):13-38.
130. Darwin M: The cryonic suspension of A-1184: http://www.alcor.org/cryonics/cryonics9208.txt. . Cryonics 1992, 13(8):9-11.
131. Henson K: Unpublished case data of Alcor patient A-1475, Stanislaw Penksa, 26 November, 1995. 1995.
132. Darwin M, Leaf, JD, Hixon, H.: Case report: neuropreservation of Alcor patient A-1068: http://www.alcor.org/cryonics/cryonics8504.txt. Cryonics 1986, 7(2):17-32.
133. Darwin M: Jerry Leaf enters cryonic suspension: http://www.alcor.org/cryonics/cryonics9109.txt Cryonics 1991, 12(9):19-25.
134. Darwin M: Cryopreservation case report: Arlene Francis Fried, A-1049: http://www.alcor.org/Library/html/fried.html. In.: Alcor Life Extension Foundation; 1995.
135. Darwin M: Cryopreservation of James Gallagher, CryoCare patient #C-2150: http://www.alcor.org/Library/html/casereportC2150.htm. In.: Alcor Life Extension Foundation; 1995.
136. Darwin M: Unpublished case report of Alcor Life Extension Foundation patient A-1410, 27 July, 1992. 1992.
137. Bridge S: The cryonic suspension of Alice Black. Cryonics 1988, 9(11):15-25.
138. Tulving E: Episodic and semantic memory. In: Organization of Memory. Edited by E Tulving WD. New York: New York: Academic Press; 1972: 381–403.
139. Gabrieli J, Kao, Y.: Development of the Declarative Memory System in the Human Brain. Nature Neuroscience 2007, 10:1198-1205.
140. Eichenbaum H: A cortical-hippocampal system for declarative memory. . Nature Reviews Neuroscience 2000, 1:41-50.
Selected Bibliography of Full Text PDFs
Brain Ultrastructure and LTP:
Harris KM (1980) Relationships between dendrite and spine neck diameters in freeze-fractured rat hippocampal formation. Biol. Bull. 159:470-471. (646K PDF)
Harris KM, Landis DM (1986) Membrane structure at synaptic junctions in area CA1 of the rat hippocampus. Neurosci. 19:857-872. (3,253K PDF)
Harris KM, Stevens JK (1989) Dendritic spines of CA1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 9:2982-2997. (7,143K PDF)
Chicurel ME, Harris KM (1992) Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and theirsynaptic relationships with mossy fiber boutons in the rat hippocampus.J. Comp. Neurol. 325:169-182. (6,353K PDF)
Sorra KE, Harris KM (1993) Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and theirtarget pyramidal cells in hippocampal area CA1. J. Neurosci. 13:3736-3748. (5,414K PDF)
Harris KM, Sultan P (1995) Variation in number, location, and size of synaptic vesicles provides an anatomical basis for the non-uniformprobability of release at hippocampal CA1 synapses. J.Neuropharmacology34:1387-1395. (851K PDF)
Spacek J, Harris KM (1997) Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendriticspines of the immature and mature rat. J. Neurosci. 17:190-203. (2,560K PDF)
Spacek J, Harris KM (1998) Three-dimensional organization of cell adhesion junctions at synapses and dendritic spines in area CA1of the rat hippocampus. J. Comp. Neurol. 393:58-68. (947K PDF)
Shepherd GMG, Harris KM (1998) Three-dimensional structure and composition of CA3–>CA1 axons in rat hippocampal slices: implicationsfor presynaptic connectivity and compartmentalization. J. Neurosci. 18:8300-8310. (964K PDF)
Sorra KE, Fiala JC, Harris KM (1998) Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampalsynapse formation. J. Comp. Neurol. 398:225-240. (1,643K PDF)
Ventura R, Harris KM (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neuroscience 19(16):6897-6906. (888K PDF)
Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC (2002) Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J. Neurosci 22:2215-2224. (763K PDF)
Harris KM, Cruce WLR, Greenough WT, Teyler TJ (1980) A Golgi impregnation technique for thin brain slices maintained in vitro. J. Neurosci. Methods.2:363-371. (3,633K PDF)
Sorra KE, Harris KM (1998) Stability in synapse number andsize at 2 hr after long-term potentiation in hippocampal area CA1. J. Neurosci.18:658-671. (1,407K PDF)
Kirov SA, Sorra KE, Harris KM (1999) Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci. 19(8):2876-2886. (1,426K PDF)
Ventura R, Harris KM (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19(16):6897-6906. (888K PDF)
Sorra KE, Harris KM (1998) Stability in synapse number and size at 2 hr after long-term potentiation in hippocampal area CA1. J. Neurosci.18:658-671. (1,407K PDF)
Sorra KE, Fiala JC, Harris KM (1998) Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampalsynapse formation. J. Comp. Neurol. 398:225-240 (1,643K PDF)
Kirov SA, Sorra KE, Harris KM (1999) Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neuroscience 19(8):2876-2886. (1,426K PDF)
Kirov SA, Harris KM (1999) Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nature Neuroscience 2(10):878-883. (643K PDF)
Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC (2002) Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J. Neurosci. 22:2215-2224. (763K PDF)
Fiala JC, Allwardt B, Harris KM (2002) Dendritic spines do not split during hippocampus LTP or maturation. Nat. Neurosci. 5:297-298. (311K PDF)
Ostroff LE, Fiala JC, Allwardt B, Harris KM (2002) Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron 35:535-545. (552K PDF)
This website is a superb resource for visual information as well as full text papers on the fine structure of the mammalian central nervous system:
http://synapses.clm.utexas.edu/lab/lab.stm
http://synapses.clm.utexas.edu/anatomy/chemical/synapse.stm
Vitrification:
Fahy, GM, Wowk, B, et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology 48 (2004) 157–178: http://www.21cm.com/pdfs/cryopreservation_advances.pdf
Fahy, GM, Wowk, B, Wu, J. Cryopreservation of Complex Systems: The Missing Link in the Regenerative Medicine Supply Chain. Rejuvenation Research. 2004, 9(2):279-91: http://www.21cm.com/articles/Missing_Link.pdf
Fahy G, Wowk, B, Pagotan, R, et al.: Physical and biological aspects of renal vitrification. Organogenesis 2009, 5(3):167-175: http://cryoeuro.eu:8080/download/attachments/425990/FahyPhysicBiolAspectsRenalVitri2010.pdf?version=1&modificationDate=1285892563927
Wowk B: Anomalous high activity of a subfraction of polyvinyl alcohol ice blocker. Cryobiology 2005, 50(3):325-331: http://www.21cm.com/pdfs/anomalous.pdf
Pichugin Y, Fahy, GM, Morin, R.: Cryopreservation of rat hippocampal slices by vitrification. Cryobiology 2006, 52(2):228-240: http://www.21cm.com/pdfs/hippo_published.pdf
Brain Cryopreservation by Freezing:
Darwin, M, Russell, S, Wakfer, P, Wood, L, Wood, C, Effect of a human cryopreservation protocol on the ultrastructure of the canine brain. (Originally published by BioPreservation, Inc., as BPI Tech Brief 16 on CryoNet and sci.cryonics, May 31, 1995): http://www.alcor.org/Library/html/braincryopreservation2.html and http://www.alcor.org/Library/html/braincryopreservation1.html.
Darwin M, Leaf, JD.: Cryoprotective perfusion and freezing of the ischemic and nonischemic cat: http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1389, http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1390, http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1391, http://www.cryonet.org/cgi-bin/dsp.cgi?msg=1392 See also: Federowicz, MG. and Leaf JD. Cryonics. issue 30, p.14,1983.
Suda, I., Kito, K, Adachi, C. Viability of Long Term Frozen Cat Brain in Vitro. Nature. v. 212, Oct. 15, p. 167:1966: http://cryoeuro.eu:8080/download/attachments/425990/SudaNature1966.pdf.
Suda, I., Kito, K, Adachi, C. Bioelectric discharges of isolated cat brain after revival from years of frozen storage. Brain Res. 70;527-531:1974: http://www.ncbi.nlm.nih.gov/pubmed/5970120?dopt=Abstract. Retrieved 2010-08-31
[1]The resolution of direct digital imaging technology using charge-coupled devices (CCDs) is still well below that pf the best fine grain photographic films – color or black and white.
[2] For instance, does a person remain the ‘same’ person after head injury that causes major and permanent changes in personality or in cognitive ability? Or, alternatively, does a person ‘survive’ brain injury that leaves his personality and cognitive capabilities intact, but which deprives the individual of some or all of his memories? If permanent amnesia does occur, will the loss of some memories prove identity critical, whilst the loss of others be merely inconvenient, but not represent a fundamental compromise to personal identity?
[3] Exposure to environmental toxins such as mercury or to psychoactive drugs may also permanently alter cognition and personality in adults.
[4] By interacting with water so strongly via hydrogen bonding CPAs may also make the remaining water less biological available to perform the solventing and stabilizing functions it normally provides.
[5] Biologists arguably ‘know better’ in that they presumably have a better grasp of the fluid nature of cell and cell component morphology.
[6] Ischemia is absent or inadequate blood flow to tissues as occurs in cardiac arrest, heart attack and stroke.
[7] Rather, such a configuration is a product of conscious design at the meta-level.
[8] The outer lamina of the cerebral cortex, containing the neuronal soma and dendrites of Purkinje cells, the axons of the granule cells, and the cell bodies, dendrites, and axons of basket cells.
[9] Declarative memory (sometimes referred to as explicit memory) is one of two types of long term human memory. It refers to memories which can be consciously recalled such as facts and events.[1] Its counterpart is known as non-declarative or Procedural memory, which refers to unconscious memories such as skills (e.g. learning to ride a bicycle). Declarative memory can be divided into two categories: episodic memory which stores specific personal experiences and semantic memory which stores factual information.

you wrote:
“Cryonicists may wish to rethink the way they communicate the viability of cryonics to the public ….if they realistically hope to benefit from cryopreservation as a potentially reversible method of medical time travel.”
How in the heck is the way we “communicate the viability of cryonics to the public” going to help us “benefit from cryopreservation”?
The ” … ” are yours, not mine. Sorry if my meaning was not clear. What I wrote was: “Cryonicists may wish to rethink the way they communicate the viability of cryonics to the public – as well as how they conduct their personal lives – if they realistically hope to benefit from cryopreservation as a potentially reversible method of medical time travel.” And what I meant was:
1) Adequately informing people about cryonics who are young and healthy, or who are at least not dying or desperate, is a really tough problem. Providing informed consent to people who are in at-need situations is practically impossible. I say “practically,” because there are some people who call after the last minute who have had prior extensive contact with cryonics, or who are otherwise very well informed. But this is rare. Most people who call after the medico-legal death of a loved one are not only not adequately informed, usually they cannot be adequately informed – at least not in the time frame available. By way of analogy, if a surgeon informs me that he believes it is necessary to amputate my gangrenous hand in order to save my life, I am, at that point, pretty well informed enough to make a decision. However if a neurosurgeon tells me he wants to cut out part of my brain – well, that’s a whole different can of worms. You can bet I am going to carefully consider my options, including whether or not “I” will still be there after the surgery is over. I’ve had YEARS of considering questions like this, and it would still take me a good while, and a lot of research and reading, before I could make an informed decision.
Deciding on cryonics isn’t like buying a toaster, or even getting your hand amputated. It’s complicated, and it involves a lot of subtle considerations. This is hard enough when a person is making a decision for themselves. It becomes much more problematic when making it for someone else, especially if the decision impacts the lives and finances of others. When you start to treat cryonics as a business enterprise where people present strictly as “customers,” then at very least you have a commonsense, self-interested “obligation” to protect the customer from himself under circumstances where he is vulnerable, and at a disadvantage. If you fail to do this, you will suffer grievously in the long run, and the government will do it for you. Cigarette companies not only used to promote cigarettes to children (I used to buy boxes of Camel and Marlboro cigarette chewing gum, as a kid), they actually passed them out in schools prior to WWII. The kids would come into the classroom and there would be a box of coffin nails on their desks.
2) Cryonicists who want to get more out of cryonics than ritual reassurance should realize that they MUST all but abolish ischemia. That realization will have all sorts of practical implications. Mostly though it should lead to a rethink of how we lead our lives, because if we expect we can just get on with our business and count on “Remote Standby,” we’re just kidding ourselves. A favorable microenvironment for cryonics must be created and inhabited. — Mike Darwin
A favorable microenvironment for cryonics must be created and inhabited.
I think what this means is that we have to establish our own medical facilities such as clinics and nursing homes staffed with medical professionals that are either of our own or are sympathetic to cryo-preservation.
I’m still working through this post. Got through about a third of it yesterday. I still have a lot of definitions to look up.
In the meantime, an offtopic question for this post: in your recent manifesto, you talk about changing the basis for organizing cryonics as an enterprise, suggesting that we think of other models than just capitalistic industries, and pointing to secret societies such as the Masons who have endured centuries and survived the fall of nation states in the shroud of secrecy conducting their own affairs.
Some people are interpreting you to be saying that cryonics should be turned into a secret society or cult. Is this really how you feel about it?
Also, have you considered posting the manifesto and some of your other recent writings here on chronosphere?
Thank you very much for asking those questions. That was exactly what the “manifesto” was supposed to do; cause people to ask questions, and more importantly, start a dialogue on the issues raised in it. I can’t give a full answer to all of your questions, because no such answer yet exists. That will require an effort on the part of a number of people, and ultimately it will require the efforts and ongoing work of a community of people.
Which brings me to a question I can answer (definitively) providing we can agree on the definition of a what a ‘cult’ is. Right now in the West, “cult” is a pejorative word which has all sorts of bad implications – and that’s the definition I’m going to assume is in operation here. Cults are generally understood to have these characteristics (at a minimum):
1) People are put in physical or emotionally distressing situations;
2) Their problems are reduced to one simple explanation, which is repeatedly emphasized;
3) They receive unconditional love, acceptance, and attention from a charismatic leader or group;
4) They get a new identity based on the group;
5) They are subject to entrapment (isolation from friends, relatives and the mainstream culture) and their access to information is severely controlled.
Per point #1, above, I don’t have to do anything to “put people in physical or emotionally distressing situations,” since they are already in that situation if they are cryonicists. If you think cryonics is a good idea, you already have anxiety issues, and there is nothing I can do to make them worse ;-). And if you’ve seen any simple explanations here, well then you’re vastly smarter than me, because all I see is a lot of complex problems with no easy solutions. And you sure as hell aren’t going to get any one simple explanation from me to solve them. As to unconditional love and acceptance, well if you want that you can join CI, I suppose. I’ve been accused of all kinds of things, but NEVER of giving unconditional love and acceptance, and I don’t foresee me mending my ways, in this respect. Here’s the paradox, just go back to Cryonics magazine from day 1 till 1991, and see what you find there. Then go to the Internet and gather up all my Cryonet posts. READ THEM, or a representative sample. Next, do the same for Alcor after 1991, for CI, and for any other personality you choose in cryonics. If there is anything cult-like (per above) in that material or in my actions over the past 3+ decades, I would be most surprised. Alcor had a non-discrimination policy under my watch, a very high standard for informed consent (in fact I introduced the idea into cryonics!) and a broad outreach to an equally broad spectrum of people. It was a dynamic and transparent place compared to any cryonics operation now in existence, bar none. My fights were more often with fellow Officers and Directors over releasing information, not concealing it. The problem with cults is that they are inherently fragile until they become religions – and they are miserable places to live. I know, because I was in one for a year. In fact, they are exactly the kinds of places that much of cryonics has now become, where you are “in” or “out” and must constantly worry about what you say and do, lest you be on the wrong side of the next purge. Finally, look at the information starting to accumulate here. Where is its equal elsewhere? And when you find it, who wrote it? Me, or one of the people now sneeringly referred to as my “proteges,” such as Brian Wowk or Max More.
My identity and my name are my own – I chose them as much as any man can. I don’t much care what other people call themselves, and I sure don’t think it’s a good idea for anyone else to try to decide that for them. And as to the Extropian/TH fashion of changing birth names, that was an FM-2030 thing; I have never remotely suggested that someone change their name, let alone their identity. And I find the idea of cutting people off from friends and family utterly repugnant. I’ve seen plenty of that from the other side, as a consequence of being a gay man. Cripes, I only wish I could have given a lot of the people who’ve shown up in cryonics over the years new identities. And believe me, if I could have, they wouldn’t be anything like so many of them are now…
So, in the sense outlined above, I certainly do not think cryonics should be turned into a cult. I’d be the first one thrown out of it.
Secrets, well yes, you bet. There are two kinds of enterprises in this world – those that have secrets, and those who’ve spilled some of them. A global riptide of political upheaval has just been nucleated by Wikileaks – and the interesting thing is that almost all of the incendiary material that has ‘leaked’ is not even highly classified, or nor truly secret. That makes me wonder what the real secrets are like – the things that are classified.
I want the people pouring liquid nitrogen on me to have some kind of hard and fast commitment – beyond a paycheck. Hell, my physicians (mostly) swear an oath that sets out the basic conditions under which they will care for me. I want a frigging blood oath for the people entrusted with my cryonics care ;-). And I’m not at all shy about saying that I don’t think anything short of what we had in Alcor in 1987 is going to work. But, that’s a bit a writing that requires an accompanying picture or two, so I’ll save it for later.
Having said that, if you want to point to the Masons or the Moonies or the Scientologists and say, “There! There it is, that’s what we should do! That’s what we should be!” well, you missed my point altogether. By definition, we need something new, something that has not previously existed because we have not previously existed before. That means that we have the pleasure and the peril of trying craft a mechanism that will work for us and meet our needs. I don’t know how to do that de novo, starting from scratch, and neither does anyone else. We are still very much human beings, and we will still largely choose from the menu of coping strategies developed by other human institutions. There’s no sin in that. In fact, what we will look for and use is undoubtedly mostly what other institutions have used to cope with similar problems, in the past.
As to capitalism, I love capitalism for what it is, a tool and a means, not an end. Corporations exist to serve us, not the other way around. And the business corporation, a la the Santa Fe Railroad, US Steel, or Apple, is most definitely not the entity that will serve to transport cryonicists into the future. Why this should be an issue for debate eludes me, since there are no such patient care cryonics corporations left. A business corporation is in the business of business, and none of us is rich enough to pay for what it will take to transport us through time, and pull our frozen keesters out of LN2 and restore them to life. — Mike Darwin
This is a very nice paper!
You might want to put this in pdf format, along with your previous ischemic injury postings.
Another issue involving ischemic injury is the morbid state of the person being cryopreserved. Cardio-vascular disease and diabetes is going to reduce the efficacy of perfusion even in ideal medical-legal situations. Blocks arteries in the brain will certainly prevent the effective circulate of cryoprotectants and make for a poor suspension. A life style change that reduces the risk of cardiovascular disease and diabetes, even at the expense of increased risk of non-circulatory conditions, might not be a bad idea for those seeking cryo-preservation.
Also, you are saying that too high concentration (too little water) also causes damage by denaturing proteins and other macro-molecules, thus causing damage to membranes and other cellular structures. This suggests a therapeutic window of optimal concentration of cryo-protectants. This therapeutic window likely varies between tissues, which requires that one prioritized the cryo-preservation of the brain over all other tissues and organs.
PDFs: I’m squashed with work right now, and trying to get prepared to go to Europe for some months, so it isn’t going to happen soon. I do want to pour the pdfs of these articles on line for many reasons. For one thing, I want as much durability and dissemination for historical purposes, as possible. I am a highly visual person, but personal aesthetics aside, there is enormous information in the photographs that are being posted here. The image URLs will eventually expire, and the life and color will go out of the articles with them. PDFs provide some defense against such losses.
The presence of atherosclerosis doesn’t really impact perfusion unless it has caused virtually complete occlusion of a critical vessel. This is usually an acute process, and occurs when a plaque ruptures. Otherwise, if the vessel is open to flow at the time of cardiac arrest it will usually provide adequate flow. One problem which can be quite serious that is directly related to atherosclerosis is the inability to get “rapid” vascular access due to extensive plaque formation in the femoral arteries. The artery lumen may be so narrowed that a cannula cannot be placed, or much worse, the cannula may be placed between the atheroma and the the muscular wall of the vessel (the tunica media vasorum). When flow is initiated, the interior (intima) of the vessel is dissected away all the way up the arterial tree! I’ve seen a femoral dissection run from the groin all the way to the basilar arteries in the brain. Under such conditions, you cannot perfuse at all. This kind of intimal dissection can also occur if the plaque is nicked or destabilized at, or beyond the tip of the cannula. In short, it’s a disaster, and yes, it happens clinically in cardiopulmonary bypass, and sometimes spontaneously in the course of aortic aneurysms. The TV actor John Ritter died from a thoracic aortic dissection.
The lifestyle changes I’m speaking of are to rearrange how, and very likely where we cryonicists live our lives, in order reduce the risk of ischemia. There are people who say cryonics hasn’t ‘failed,’ and my response to that would be to point out that if there were 1000,000 or even 10,000 cryonicists, the issue of ischemic time would be much less pressing. As it is, it is an intractable problem for most cryonicists and they deal with it mostly by simply not knowing about it (the consequences, that is) or minimizing its impact if they do. That’s a major reason I just wrote a mini-e-book about it.
CPA Toxicity: You’re a scientist, so I’ll mostly refer you to the literature to read for yourself. I will say that by any normal frame of reference, the CPAs being used for cryoprotection in cryonics and cryobiology are ASTONISHINGLY non-toxic. I mean, think about it, how many compounds do you know of that you could replace 55% of the water in highly metabolically active tissues, like the brain or the kidney, and have them recover 100% viability – and in fact, do a little better than the controls, that were simply sitting in organ preservation solution in cold storage during the testing interval! And yes, it is almost certainly related to water removal from the solution, because what you see (over and over) is that a very small difference in concentration of CPAs causes a large loss of viability. Thus, you can be just fine at 55% and have a huge reduction of viability at 57%. For M-22, if I recall correctly, the critical threshold is in the vicinity of 60%. And of course, the temperature of introduction is critical. The final loading of M-22 is done at -20 deg C for kidneys!
As to the tissue specificity of CPA toxicity, that’s not my area of expertise, and the people who would be able to give the most accurate answers are not going to tell me before they publish. I will say that based on the published/disclosed data about M-22, it seems to equally toxic (or benign) to brain and kidney. On the other hand, organs are incredibly picky about the carrier or preservative solution they are stored in under ultraprofound hypothermic conditions. Hearts don’t do well with kidney preservation solutions like Viaspan, and lungs need something else altogether. The big divide is between muscle and everything else – and I suspect brains may be in the same camp as muscle. Muscle is frustratingly difficult to store under cold ischemic conditions (blood washed out & stored on ice). It goes into cold rigor (mortis), usually within 4-6 hours of the start of cold ischemia. This is why the preservation time is so short for adult hearts for transplant. Pedes’ hearts and infants’ hearts can often go to 12 hours, and sometimes longer.
What do you have in mind when you talk about “life-style” changes for cryonics people?
One activity that comes to mind is establishing our own medical clinics and nursing homes for members approaching deanimation. Of course, there will be push-back from the rest of the medical community and we will have to be political adept to resist and survive this push-back. The medical system, especially the MD’s in the AMA, is essentially a medieval guild and they will fight anything that they perceive as threatening to their monopoly power. The horror scenario we want to avoid is where our clinic in accused of “offing” patients in order to avoid ischemic injury, essentially a repeat of Dora Kent.
Secret societies are a useful model, as is the Catholic Church. Being a secret society is not the same thing as being a cult. Free masonry is no cult, but is an effective secret society. As far as defeating aging, cryonics is a 200-300 year venture. Of course we will need cryonics even after we defeat aging, but post-mortals ought to be more receptive to cryonics (or at least tolerant of it) than current people.
What do you mean by this: “As to unconditional love and acceptance, well if you want that you can join CI, I suppose.”
It was a snotty, and probably inappropriate remark. Basically I was commenting on the operational paradigm at CI, which is pretty much “ritual.” You sign up, you get frozen and it’s pretty much kumbaya, no matter how badly things go. And they go pretty badly. Go to: http://cryonics.org/refs.html#cases and start reading the case reports posted there. That’s pretty much my working definition of horrible. It seems apparent to me that “just getting frozen” is now all that is necessary for a ticket to tomorrow, and that anything else that is done is “just gravy,” and probably unnecessary to a happy outcome.
It works like this:
1) If you are cryopreserved, hope is preserved that you will be reanimated.
2) Since hope cannot be quantified, all hope is equal.
3) Since the degree of hope is the same for everyone cryopreserved everyone cryopreserved has the same opportunity to be reanimated.
4) Since being cryopreserved equals salvation, then how you are cryopreserved is no longer material.
5) People who get cryopreserved ‘well’ have the right to more hope, however, since hope cannot be quantified this is meaningless.
Even in cases that CI perfuses, things go horribly wrong – often – and usually for to me bizarre and unfathomable (and careless) reasons. My dear friend and mentor Curtis Henderson was little more than straight frozen because CI President Ben Best had this idea that adding polyethylene glycol to the CPA solution would inhibit edema. Now the thing is, Ben had been told by his own researchers that PEG was incompatible with DMSO containing solutions, and resulted in gel formation. Nevertheless, he decided he would try this out on Curtis Henderson. He did NOT do any bench experiments, or do test mixes of solutions, let alone any animal studies to validate that this approach would in fact help reduce edema (it doesn’t). Instead, he prepared a batch of this untested mixture, and AFTER it gelled, he tried to perfuse Curtis with it. See my introduction to Thus Spake Curtis Henderson on this blog for how this affected me psychologically and emotionally. Needless to say, as soon as he tried to perfuse this goop, perfusion came to a screeching halt. They have pumped air into patient’s circulatory systems… I could go on and on, but all you need to do is really look at those patient case reports and think about everything that is going on in those cases critically.
Ethics aside, it is CI’s policies, structure and way of handling at-need patients that is causing the nightmare of straight frozen patients that have now come to predominate their case load. Years ago, I suggested an independent service that would act to respond in high quality, at need cases: stabilize/freeze the patient (7.5 M glycerol perfusion) and then hold, until the patient was cleared for long term funding. “Not practical,” I was told.
Obviously, many other solutions are possible, including behaving like Alcor did 20 years ago, and carefully assessing the situation in real time, and responding accordingly. There are many kinds of ways to quickly verify/obligate people for funding: air ambulances, and all sorts of other businesses do this all the time. What is unethical is the sleight of hand CI has engaged in. They want to be able to say that, “No cryonics patient has been thawed out for lack of funding since 19XX…” So, in order to make that so, they get the mortuary industry to freeze the poor devils, and then if things “don’t work out,” it’s the morticians who get stuck thawing the person out. It’s a beautiful “moral switch and bait” in that it recasts the act of cryopreserving a person such, that: You are not a cryonics patient when you get frozen. You are not a cryonics patient if you stay frozen for years. In fact, you are only a cryonics patient when CI says you are cryonics patient. CI has become the Hane’s Underwear, Co., Inspector #12 of cryonics.
The lack of feedback, and the wild and unbounded hand waving about what will be reversible in the future has, in my opinion, totally distorted both CI and Ben Best’s personal thinking. This has also, to a great extent, happened in cryonics as a whole. It has become a ritual that would do the Egyptians proud, and makes me recall my Catholic upbringing, and the nutty stretching of dogma that went on there, such as giving last rites to pretty long dead corpses and baptizing really long dead babies so that they could get into heaven, and not spend eternity in limbo. It is human nature in a very sad state. But, that’s just my opinion, and unconditional love and acceptance it is not. — Mike Darwin
I know this was the case when Ettinger ran things there. But I thought Ben Best was supposed to improved things at CI. Perhaps they are relying on S.A. to do all of their suspensions.
Pingback: The case against cryonics
Pingback: Longevity Medicine » Cases Against Cryonics
Pingback: Cases Against Cryonics | Longevity Medicine
Pingback: Gerald Feinberg on physics and life extension | Depressed Metabolism
Mr. Darwin…Mike…
That there are no more than 15 comments at the time of my post (the majority of which are either yours or Abelard Lindsey’s) is, I think, a profound indication of the pervasive and destructive ignorance with which humanity happily carries out its collective existence in the early 21st century.
I’ve long been familiar with the history of cryonics and your critical role in its nascent evolution, though only in the most superficial sense. Beyond recognizing the inherent worthiness of cyronics as a technological pursuit, I’d been largely unaware (and unappreciative) of the depth and breadth of the technical hurdles that have been overcome as well as those that yet remain.
I’d only ever glanced at your chronopause blog, a curiosity I considered to be the last outlet of a frustrated pioneer. Only now, after I’d set aside time to properly digest and consider the wealth of information you’ve shared here do I realize the importance of your (continuing) contributions to cyronics…and by extension, humanity.
This post in particular is an example of the astounding treasure trove of hard-won experiential knowledge inside that destined-to-be-preserved brain of yours. Shared publicly and freely for all the world to disseminate, I am firmly convinced that it will help pave the path towards a future (however far away it might be) in which the trailblazing work of pioneers such as yourself ensures a rich and rewarding existence for all of humanity. …a future in which the potential of our miraculous minds is ever more fully realized.
Since I am a layperson, and any worthwhile contribution I can make with words regarding these topics is necessarily limited, I’d just like to keep it simple and offer you my sincere and heartfelt thanks. I’m sure that you’ve long ago realized that your actions constitute an essential link in an unbroken chain extending into the far future, a future in which many, many lives will benefit.
For whatever it’s worth,
Thanks Mike.
(a 30-year-old aspiring cryonicist)