By Mike Darwin
This post is in response to a comment made by Abelard Lindsey on 02-27-2011. Since my response necessitates the use of illustrations, I am making it here, rather than in the comments section.
| Abelard Lindsey kurt2100kimo@yahoo.com.tw 71.236.250.197 |
If Melody Maxim thinks the existing organizations are so screwed up, she is free to get up off her ass and start a new organization that will do things the way she thinks they should. It’s easyto sit on the sidelines and to criticize the efforts of others. It is more difficult and takes more character and work ethic to get up off of one’s ass and to actually do some effort to make things better. The ball is in Melody Maxim’s court. Its time for her to put up or shut up. |
______________________________________________________________
You know, the ironic thing is, when Maxim first arrived on the scene, I was really pleased, and I looked forward to being able to work with her in some capacity. I had dealt with a number of perfusionists before, and in fact employed two perfusionists when I operated BioPreservation, a cryonics service provider company in the 1990s. My communication with Maxim was necessarily constrained when she was first hired by Suspended Animation, Inc., because I’d learned from previous experience that it is essential to let people gain their own unbiased experience of an enterprise, and especially of a new employment situation, before any negative input is given. Not only is criticizing someone’s employer under such circumstances considered sleazy behavior, it also just doesn’t work. You are perceived as having an axe to grind, being jealous, and so on.
Additionally, my first introduction to her had come in the form of an unexpected phone call from Charles Platt, who put Maxim on the phone to “meet” me. It was a brief (and from my end) awkward conversation. It would have been out of place for me to have then contacted her to criticize SA. I figured she’d come to her own sorry conclusions in due time, and if she was competent, it would be in a short period of time. Subsequently, Charles provided her with a copy of an extensive critique of SA that I had done as a favor (unpaid) to Bill Falloon. I was also asked for specific technical commentary on the in-field extracorporeal circuit for blood washout that SA was trying to develop. There, I apparently made the mistake of disagreeing with Maxim on just one point: the desirability of roller pumps (tubing pumps) over centrifugal pumps for use in such systems. This apparently angered her, and it was all downhill from there. Subsequent interactions with her (at a distance) when she was consulting for CI apparently enraged her.
Her response to my comments about centrifugal pumps in the setting of both asanguineous blood washout/recirculation and cryoprotective perfusion should have been a red flag at the time. At first glance, centrifugal pumps soundly beat roller pumps and other “positive displacement” pumps for extracorporeal support. They are lighter, provide more flow for less power (and weight), do less damage to blood cells, and, most importantly, they cannot pump “bulk” or macro-air into the patient’s circulatory system. If you empty the venous reservoir feeding a centrifugal pump, it loses prime and stops pumping. The impeller continues to spin, but there is no longer any flow. They are thus “inherently safe” in terms of pumping macro-air.
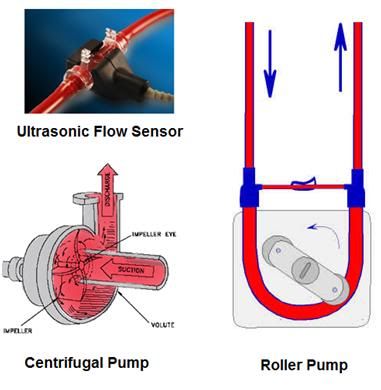
Figure 1: Centrifugal pump at left, and positive displacement roller pump, at right. Centrifugal pumps require a flow measurement device, and in medical applications this is typically an electromagnetic or ultrasonic flow meter, the transducer for which snaps around the tubing, or a special cuvette, as seen at left, above.
So, what’s not to like? When scientific-cryonics got started in the early 1970s, the standard pumps being used in extracorporeal medicine at that time were all positive displacement pumps. Mostly they were roller pumps, but some finger pumps were still in use. A typical roller pump is shown at right, above. A roller pump has two rollers that compress or “milk” the fluid in a section of tubing (the pump “shoe” or “raceway”) by either completely occluding or almost occluding the tubing (Figure 1). They will thus pump almost anything inside the tubing, including air. They also have another feature that can be deadly dangerous in extracorporeal medicine, and that is that if you occlude the outflow of the pump it will keep pumping and increasing pressure until, a) the motor is not powerful enough to continue pumping against the back-pressure, or b) the tubing and/or components in the circuit between the pump and the occlusion explode or come apart (disconnect, or “blow-off”). One other disadvantage is the action of the rollers on the tubing raceway cause it to spall, or to shed microscopic bits of plastic debris into the blood, and thus into the patient (if not intercepted by the arterial filter). These pumps, despite their seemingly overwhelming disadvantages, were the standard in cardiopulmonary bypass (CPB) until very recently. And they are still widely used – and they still predominate in the Third World.
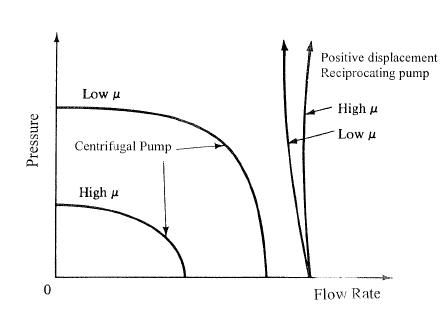
Figure 2: Comparison of flow/pressure curves of centrifugal and positive displacement pumps.
While centrifugal pumps have great safety and efficiency advantages, they have some nettlesome downsides. Chief amongst these was that a shaft to drive the impeller had to penetrate the pump housing (left in Figure 1, above) and that meant you needed a seal. This caused all sorts of problems when handling a sterile fluid like blood, and a fluid which could tolerate no contamination. Seals are a source of friction, and thus of particulates. Centrifugal pumps are also almost impossible to adequately clean, and tubing in a roller pump was disposable. Two other problems were that centrifugal pumps are notorious for causing cavitation in the fluid they are pumping. Because they can create regions of very strong negative pressure, they can cause gas bubbles to form – bubbles that subsequently embolize the patient’s circulatory system. The impeller from centrifugal pumps can also generate enormous shear and turbulence in the liquid, and these cause hemolysis and damage to platelets and white blood cells.
It took decades to design centrifugal pumps that could handle blood “atraumatically,” and when this was finally accomplished, centrifugals performed better than roller pumps under laboratory conditions (there is very little difference clinically in terms of neurological outcome). Since there is no compression of friable tubing in centrifugal pumps, there is also no spallation of plastic particles into the blood. Finally, centrifugal pumps, unlike roller pumps, do not have any rigorous correlation between the rpm of the drive motor and the flow output of the pump. Instead, flow output is a complex function of rpm, torque and back pressure – and so is the developed pressure in the system. You can see this in Figure 2, above. What this means in practice is that you must have a flow meter to actually measure the flow the pump is producing at any given time. This wasi pretty easily accomplished in industrial applications n the 1970s (and before) by using paddle-wheel, falling ball, or other kinds of in-line flow metering devices. But these solutions don’t work well, or in most cases at all, for blood.
By contrast, every turn of a roller pump will deliver a fairly predictable and uniform bolus of flow. To know your flow, all you have to do is to count the number of rpms, and multiply that number by the volume of fluid displaced with revolution of the rollers. In the early days of CPB, you had to do this by using a “look up table” to convert rmps to flow, based on the diameter of the tuning you were using in the pump head. It was simple, easy and foolproof, but could be dangerously distracting. In the late 1970s, little calculating devices with LED displays did that work for you, and look-up tables taped to the front of the pump console disappeared.

Figure 3: A magnetically-driven, seal-less, injection molded, disposable polycarbonate centrifugal blood pump head.
By the 1980s, huge advances in manufacturing technology made the magnetically-driven, seal-less, injection molded, disposable polycarbonate centrifugal pump head, similar to that shown in Figure 3, not only possible, but economical. Now, the impeller could be driven via magnetic coupling, and no shaft or seals were necessary. Cleaning wasn’t an issue because the whole thing could be thrown away after it was used. And the cost had come down to about $200 per pump head. Technological advances in “non-wetted” flow measurement made electromagnetic flow meters not only practical, but affordable. So, the flow measurement problem was pretty much solved, as well. Later, the advent of ultrasonic Doppler flow measurement technology, made flow measurement even more cost effective and accurate.
Why not use these pumps for cryopatients?!!? Unfortunately, it is a characteristic of both electromagnetic and ultrasonic flow measurement devices that the physical properties of the liquid whose flow is being measured matters. Electromagnetic flow meters only work if a solution has ions or some other magnetic field distorting property – such as being slurry of metal dust, or containing a ferromagnetic molecule… The washout and cryoprotective perfusion solutions used in cryonics have low concentrations of ions – most of the salts have been replaced with sugars or mannitol (a sugar-alcohol). What’s worse, the concentration of ions will vary wildly and dynamically as perfusion proceeds, because the salts (ions) in the patients’ tissues are being washed out. Indeed, this is why the more proper name for perfusion to do “blood washout” is called “Total Body Washout (TBW),” because it is not just the blood that is being washed out, but also, to a significant extent, the very chemical composition of the patient’s extracellular space.
Ultrasonic flow meters are intolerant of the huge changes in the micro-particle concentration and the viscosity of the perfusate that occur during TBW and cryoprotective perfusate. These flow meters are calibrated and optimized in design around blood – and both extracorporeal ultrasonic and electromagnetic flow meters caution the user that they cannot be used with blood containing less than 10 or 20% red blood cells (red cells are, of course, loaded with iron containing hemoglobin, and they scatter ultrasound). Thus, measuring flow under conditions of TBW and cryoprotective perfusion has proved an intractable problem for over 35 years.

Figure 4: The Manrise model 101 Flow Inducer developed by Fred Chamberlain, the first cryonics perfusion machine, used a Cole-Parmer., Inc., Micro Pump centrifugal pump with a magnetically driven stainless steel and ceramic pump head as the “flow inducer.” A Dwyer Instruments, Co., falling ball flow meter, an in-line analog thermometer, and a sphygmomanometer (connected to a bubble trap) was used as the pressure gauge.
Ironically, given Maxim’s vitriol over cryonicists being “ignorant of centrifugal pump technology,” the first “perfusion machines” developed for cryonics used centrifugal pumps. And that meant they had to have a flow meter. After much (costly) testing, we settled on a falling ball flow meter, because it performed relatively well across the range of viscosities of perfusates we were using. But please note, this machine was build only for open-circuit perfusion, and blood, even in very small concentrations, renders a solution opaque. If you can’t see the ball, you can’t read the flow… Also, we still had to make look-up tables for each concentration of cryoprotective perfusate to be perfused because falling ball flow meters are sensitive to liquid viscosity.

Figure 5: Extracorporeal Life Safety System (ELS) Bubble Detector and Tubing Clamps for Roller Pumps. http://www.rockymtresearch.com/els.htm
The ELS is an air in line detection safety system indicated for use on roller pump bypass circuits. If air in line is detected both automatic tubing clamps and auditory / visual alarms are activated. The device includes a third clamp for the bridge loop incorporated into roller pump circuits. The Bridge Clamp opens if the line clamps are activated to prevent excessive pressure in the circuit.
Product features include:
- Bubble detection: designed so that bubble detection sensitivity can be set down to 10 uL.
- Bridge Clamp: The bridge clamp will open the instant air is detected and the patient clamps close. This creates an open circuit to prevent pressure build-up.
- Automatic bridge-line “flash”: The bridge clamp can be set to periodically open and flush the bridge line. Duration and interval controls are located on the front panel of the device. Patient clamps remain open during bridge flash cycle.
- Manual override on all clamps (all three clamps cannot be closed simultaneously).
- Dry-coupled transducer.
- Internal battery back-up with automatic recharge.
- Compatible with any system.
- Simple controls and user interface.
The ELS device has FDA clearance and has been certified by CSA to the IEC 601-1 and UL 2601-1 standards for electrical safety.
___________________________
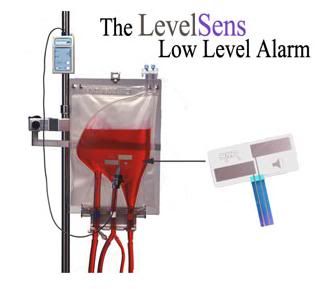
Figure 6: LevelSens, a level sensor and detector, notifies operators of low fluid levels in either flexible or rigid reservoirs. Having both medical and industrial applications, the level sensor will alarm audibly and visually when the fluid reservoir level drops below the user-placed sensor and will cease alarming when the fluid level returns. The device does not need to be reset and there is no pump interaction. http://www.rockymtresearch.com/levelsens.htm
- For Any Flexible or Rigid Fluid Reservoir.
- Functions on Both Blood and Priming Fluids.
- Reliable and Simple to Use.
- No Conflict with Other Devices.
- Increases Safety. Limits Liability.
- Powered by a 9 volt Battery.
- Self Stick, Disposable Sensors.
- CE Marked
| There are two control modules: one designed for use on rigid fluid reservoirs, one designed for flexible fluid reservoirs.
The LevelSens has FDA clearance and has been certified by CSA to IEC 601-1 and UL 2601-1 standards for electrical safety. An independent evaluation has been done by Rocky Mountain Perfusionists of Denver, Colorado. (see abstract) PRODUCTS:
|
In the intervening decades since this problem was first encountered I, and others, have repeatedly looked at the issue of how to reliably measure flow in perfusates that are dynamically varying in red cell content, ion content, cryoprotective agent content (and thus viscosity) and in temperature (which affects viscosity and often the performance of the flow meter transducer, as well). I think I have found the solution to the problem (last year, in fact), but for now, I’m keeping it to myself. And when I say found it, I mean just that – I didn’t invent it – it’s yet another artifact of technological advance inflow measurement.
One other problem with medical centrifugal pumps is that they cannot handle the very high viscosity, low flow, terminal phase of cryoprotective perfusion. The pump motor controller is not built to operate under such conditions, and the magnetically coupled impeller will, if the viscosity of the perfusate is too great, simply uncouple from the drive shaft magnet and stop turning.
Because of these issues, and especially because of the flow measurement issue, I recommended that centrifugal pumps not be used for cryonics applications at that time. Yet another reason for my doing this, and feeling comfortable with the safety issues, is that there are now rock-solid, reliable extracorporeal (FDA approved) monitoring systems for both air, overpressure, and reservoir fluid level, which will shut down the roller pump and clamp the blood conducting lines within ~ 300 msec of detecting a problem (Figure 5). The latest generation of low reservoir sensors will work on virtually any kind of plastic reservoir (hard or soft) and are insensitive to the type of fluid being used or to the temperature (down to ~ -5oC) (Figure 6). Thus, despite their disadvantages, roller pumps still trump centrifugals because it easily possible to reliably and dynamically know the flow being delivered to the patient – and that is critically important.
This is an example of an area where expertise in cryonics, related to the application of extracorporeal medicine, is critical. Medicine does not smoothly map onto cryonics, and this true not just in extracorporeal medicine, but across the board. A consequence of this is that if you try to take a physician, a surgeon, a perfusionist, or a paramedic, and simply “dump” them into the cryonics arena, they will typically make very serious errors. This is to be expected, because any specialized area of technology, be it medicine, engineering or computer programming, has its own unique operating parameters and its own unique set of specialized requirements and skills. Cryonics is properly a highly specialized branch of medicine, just as are neurosurgery and cardiac surgery. It is grossly unfair to both cryonics, and to medical professionals, to behave otherwise. I think that Maxim both refuses to believe this is so, and finds it highly offensive, neither of which change the reality of the situation.


So, Melody Maxim’s vitriol is driven by the cryonics organizations’ choice of using roller pumps instead of centrifugal pumps. Is this correct?
To be honest, I have no idea. You can read the full exchange between Maxim and myself (up until the very recent CryoNet postings) here: http://cryoeuro.eu:8080/download/attachments/425990/Maxim+-+Darwin+Cold+Filter+Exchanges+February%2C+2009.pdf
As near as I can tell, the woman has serious issues of some kind, probably unrelated to any one person or personality in cryonics. If all she were doing was posting such rants, it would be sufficient unto the day to simply post a rebuttal, and leave it at that. However, she has launched an effort to get cryonics Transport personnel banned from entering hospitals. This would have the effect of subjecting cryopatients to hours of ischemia, since SOP or Best Practice in hospitals, extended care facilities, and other licensed medical facilities is to pull all lines (and remove all tubes or invasive appliances), clean up (sponge bath) and shroud the patient (white plastic sheet wrap). Ligature of the penis to avoid urine leakage is also common practice. The patient then waits, in situ, until a morgue attendant or diener comes to make the pick up from the patient’s room/bed. Typical time from pronouncement to arrival in the hospital morgue is ~2-hours. Similarly, she has vowed to make cryonics a state regulated practice, absent any collaboration or input from cryonicists, and she is believed to be associated with these efforts: https://sites.google.com/site/cryonicsfactsheet/home/coalition-for-the-regulation-of-cryonics-1 and http://regulatecryonics.org/ She does not respond with any specific technical criticisms of my work or postings, when asked to do, so it is hard to tell what her particular objections or motivations are. — Mike Darwin
I’ve read her vitriol over the past few years. I get the impression that she is mentally ill. I have a friend who has written a medically-related book with a co-author who is also mental. His co-author’s vitriol is similar to that of “Melody Maxim”.
It is not fruitful to argue with mentally ill people.