Medical Records, Medical History and the Necessity for Highly Skilled Cryonics-Based Medical Evaluation and Judgment

“The physician must be able to tell the antecedents, know the present, and foretell the future–must mediate these things, and have two special objects in view with regard to diseases, mainly to do good or to do no harm.”[119]
-Hippocrates Of The Epidemics
By Mike Darwin
 Figure 9: Hippocrates of Kos, the father of rational medicine.
Figure 9: Hippocrates of Kos, the father of rational medicine.
In the past two decades I have had the frustrating experience of having no fewer than four Officers, Directors or Presidents of currently extant cryonics organizations dismiss the need to gather and thoroughly analyze the medical records and the medical histories of cryopatients. Two cryonics organization Presidents have informed me that these materials are not only hard to gather, but in addition, are of little or no use to the patient, since future medical diagnostic and treatment modalities will render such information superfluous at best, and misleading at worst. As a consequence, it has been deemed not merely annoying and futile to gather this information (let alone analyze it), but also a needless and costly burden on the cryonics organization, and one which is presumably best avoided.
My purpose here, from the beginning, will be not only to refute this erroneous position, but to demonstrate, largely by example from long previously published case reports, the criticality of the patient’s medical condition prior to as well as at the time he presents for cryonics care, to the quality of the cryopreservation he will subsequently receive. In addition, I wish to demonstrate that the routine acquisition and interpretation patient medical records is not just of great utility to the patient for whom it is done, it stands to benefit all patients who come later, by enriching the knowledge and experience base in the practice of cryonics as a scientific-technological discipline.
Example 1: Pre-cryopreservation Disease May Alter Critical Anatomy
The first example I am going to use to demonstrate the criticality of the patient’s medical history (Hx) and complete medical record to optimizing cryopreservation and avoiding delay took place on 12 Feb 1985. Since the involved family member discussed in this case is now in cryopreservation, it is possible to discuss the case with greater frankness than was possible when it was first reported. The patient was a 68-year-old woman (Alcor Life Extension Foundation patient A-1068) who was terminally ill with recurrent, disseminated lymphoma. The husband was not only supportive of cryonics; he was a long-time cryonicist himself. Hospital cooperation was excellent and cardiopulmonary support (CPS) was initiated within ~1-2 min of cardiorespiratory arrest and pronouncement. The patient was transported from the hospital to a nearby mortuary without any interruption in CPS and a right femoral cut-down was undertaken (with CPS still underway) to allow for femoral arterial and venous cannulation to permit femoral-femoral cardiopulmonary bypass (CPB) and total body washout. I will now quote directly from the case report as it appeared in Cryonics magazine in April of 1985:
“The patient’s right groin was prepared for surgery by swabbing with Betadine solution and draping with sterile towels and a fenestrated drape. The anatomical position of the right femoral artery and vein were located by reference to the pubic tubercle and the anterior superior iliac spine. An incision with a #10 scalpel blade was made at the midpoint between these two structures, beginning at the inguinal ligament and running parallel to the longitudinal axis of the leg for approximately 5 cm.
The femoral artery was promptly identified and an 18 fr. arterial cannula, USCI type 1860, introduced through an arteriotomy and secured with silk ties. Despite extensive dissection which consumed nearly an hour, the right femoral vein could not be located. It was later discovered from the patient’s medical records (which were unavailable at the time of transport) that the patient had a history of thrombophlebitis with a venogram done in December, 1975 demonstrating extreme deep vein thrombosis of the right leg, including the entire right femoral vein.
Owing to lack of success identifying the right femoral vein, the left groin was prepared for surgery and the left femoral vein was promptly raised and cannulated with a 32 fr. venous cannula, USCI type 1967.” http://www.cryonics.com/Library/html/casereport8504.html
The delay, due to our inability to find a non-existent femoral vessel under very difficult field conditions (the wound rapidly filled with blood-tinged interstitial fluid), occurred because the patient was massively edematous due to hepatorenal syndrome (liver and kidney failure) and fluid resuscitation (~20 L of IV crystalloid), compounded by the necessity to make a contralateral wound and raise the left femoral vein, was approximately ~ 30-45 minutes. While the patient was receiving closed chest CPS, such support (using the conventional CPR technique available at the time) was only marginally effective, and was further compromised by the presence of fulminating pulmonary edema, which greatly reduced the ability of the lungs to provide gas exchange.
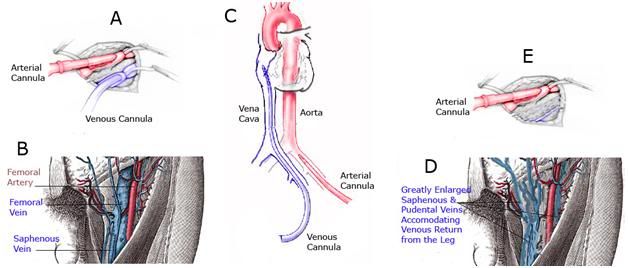 Figure 10: Femoral-femoral cardiopulmonary bypass (CBP) requires the placement of a long, large caliber cannula through the femoral vein (A&B) and into the inferior vena cava, preferably threaded up to the level of the right atrium (C). If the femoral vein has been obliterated, sclerosed, or is otherwise too small to accommodate the venous cannula (E), then either the contralateral femoral vein must be used, or venous drainage may be accomplished by cannulation of the internal jugular vein in the neck (not shown).When the femoral vein is destroyed as a result of disease or trauma, it is ‘replaced’ by a network of smaller caliber collateral vessels which serve to provide venous return from the leg to the heart (D). These vessels are often tortuous, are not of sufficient diameter to accommodate the venous cannula, and do not provide a straight, large diameter path to the inferior vena cava. In the case of patient A-1086 the arterial cannula in place in the right groin was used for perfusion (E) and the venous return cannula was placed in the left femoral vein.
Figure 10: Femoral-femoral cardiopulmonary bypass (CBP) requires the placement of a long, large caliber cannula through the femoral vein (A&B) and into the inferior vena cava, preferably threaded up to the level of the right atrium (C). If the femoral vein has been obliterated, sclerosed, or is otherwise too small to accommodate the venous cannula (E), then either the contralateral femoral vein must be used, or venous drainage may be accomplished by cannulation of the internal jugular vein in the neck (not shown).When the femoral vein is destroyed as a result of disease or trauma, it is ‘replaced’ by a network of smaller caliber collateral vessels which serve to provide venous return from the leg to the heart (D). These vessels are often tortuous, are not of sufficient diameter to accommodate the venous cannula, and do not provide a straight, large diameter path to the inferior vena cava. In the case of patient A-1086 the arterial cannula in place in the right groin was used for perfusion (E) and the venous return cannula was placed in the left femoral vein.
The patient’s husband was, at his and the patient’s request, present during the entire procedure, and he was able to inform us, as we struggled futilely to identify the right femoral vein in the massively edematous tissue, that his wife had suffered severe deep vein thrombophlebitis of the right leg, six years earlier, in 1975. This was, in fact, the reason for our inability to identify and raise the right femoral vein; it no longer existed. Our only choices in this situation were either to cut-down, raise and cannulate the femoral vein in the opposite leg, or to cannulate the internal jugular vein in the neck. Because we needed to maintain the patency of the jugular veins for drainage during CPA perfusion, we chose to surgerize the contralateral leg and cannulate the opposing femoral vein to accomplish venous drainage for CPB.
When the patient’s body was subsequently autopsied (she was a neuropatient) we found a narrow band of tissue tightly adherent to the fascia of the femoral sheath, which was all that remained of the femoral vein. What made this incident particularly difficult for all involved, was that both Jerry Leaf and I had repeatedly made efforts in the months preceding the patient’s arrest to obtain her medical records – efforts which were unsuccessful. The patient’s husband was a highly intelligent man, and he immediately understood the implications of his inaction in obtaining the patient’s medical records prior to her cryopreservation. His wife had suffered an additional ischemic insult that was completely avoidable and we missed the last plane back to Alcor by less than hour, exposing the patient to ~ 8 hours of additional cold ischemic injury as a consequence.
Example 2: Pre-Cryopreservation Pathology may Preclude or Delay Adequate Cryoprotective Agent Equilibration in the Brain without Effective Intervention
In clinical medicine, the patient’s medical history is defined as a written summary of past and present medical conditions which may contain clues bearing on their health; past, present, and future. The medical history is thus an account of all medical events and problems a person has experienced (including psychiatric illness), and it is especially helpful when a differential diagnosis is needed. By contrast, the patient’s medical record is a chronological written account of a patient’s examination and treatment that includes the patient’s medical history and complaints, the physician’s physical findings, the results of diagnostic tests (including copies of any medical imaging performed), procedures, medications and therapeutic interventions. In short, the patient’s medical record ideally contains not only all of the conclusions and summaries of a patient’s illnesses, preventative care and treatment, but also all of the raw data upon which those actions and conclusions were based.
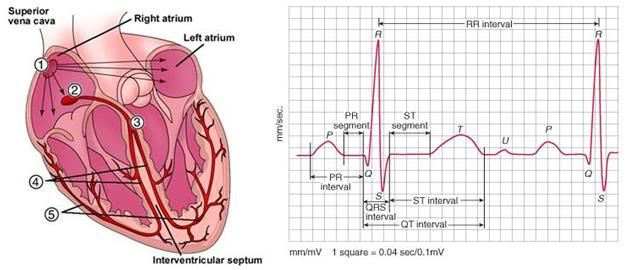 Figure 11: The anatomy of a heartbeat. The heart’s pacemaker is the sinoatrial (SA) node, which sends impulses to the atria and to the atrioventricular (AV) node, from which they are relayed to the ventricles. The signal from SA node (1) is seen as the P-wave on the ECG, at right. This signal results in the contraction of the atria, and within milliseconds it reaches the AV node (2), which in turn signals the rest of the cardiac conduction system to initiate ventricular contraction (3, 4, 5). The QRS complex of the ECG reflects the rapid depolarization and contraction of the right and left ventricles. Since the ventricular muscle mass is large, the amplitude of the electrical signal generated is on the ECG is corresponding large, as well.
Figure 11: The anatomy of a heartbeat. The heart’s pacemaker is the sinoatrial (SA) node, which sends impulses to the atria and to the atrioventricular (AV) node, from which they are relayed to the ventricles. The signal from SA node (1) is seen as the P-wave on the ECG, at right. This signal results in the contraction of the atria, and within milliseconds it reaches the AV node (2), which in turn signals the rest of the cardiac conduction system to initiate ventricular contraction (3, 4, 5). The QRS complex of the ECG reflects the rapid depolarization and contraction of the right and left ventricles. Since the ventricular muscle mass is large, the amplitude of the electrical signal generated is on the ECG is corresponding large, as well.
For example, a cardiologist in 1998 may have correctly concluded, based upon a 12-lead electrocardiogram (ECG), that a patient suffered a serious myocardial infarction (MI) of the posterior wall of the left ventricle, with evidence of a previous infarction of the left anterior descending coronary artery. However, at that time, sophisticated algorithms for analysis of the ECG were not in common use that would, for instance, allow for evaluation of subtle variations in R-R regularity of the ECG following an MI.[120, 121] While the elapsed time (interval) between R waves in the ECG appears superficially regular, careful analysis reveals that it is in fact variable, and obeys the laws of chaos theory.[122] Children show complexity and fractal correlation properties of the R-R interval time series comparable to those of young adults, whereas healthy older people demonstrate R-R interval dynamics showing higher regularity and altered fractal scaling that are consistent with a loss of complex variability.[123]
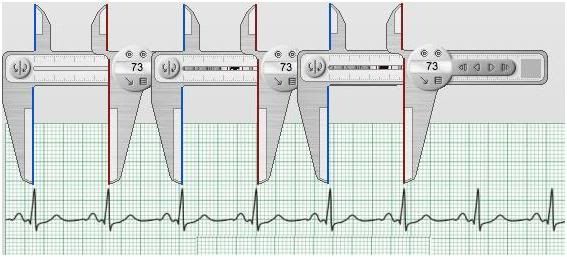 Figure 12: Loss of chaotic variability and accompanying increase of the R-R interval is a sign of damage to the cardiac conduction system which is prognostic of a markedly increased risk of sudden cardiac arrest (SCA).Simplification of the fractal complexity of the R-R interval occurs in normal aging, independent of frank cardiac disease, and may be responsible for some of the increased risk of SCA associated even with so-called “healthy aging.” Loss of R-R interval fractal complexity in the presence of cardiac disease, or during the agonal process in slowly dying patients, is prognostic of increased risk of cardiac arrest, or impending cardiac arrest in the agonal patient.
Figure 12: Loss of chaotic variability and accompanying increase of the R-R interval is a sign of damage to the cardiac conduction system which is prognostic of a markedly increased risk of sudden cardiac arrest (SCA).Simplification of the fractal complexity of the R-R interval occurs in normal aging, independent of frank cardiac disease, and may be responsible for some of the increased risk of SCA associated even with so-called “healthy aging.” Loss of R-R interval fractal complexity in the presence of cardiac disease, or during the agonal process in slowly dying patients, is prognostic of increased risk of cardiac arrest, or impending cardiac arrest in the agonal patient.
This phenomenon is very pronounced in patients who have suffered MIs, or other cardiac damage that destroys or degrades the robustness (complexity) of the system of nerves that conducts the contractile impulses to the various parts of the heart (the cardiac conduction system). As the conduction system is pruned down by age and/or disease, there are fewer and fewer signal pathways for the Q-wave, the impulse that initiates heart beat, to travel through and to reach the myocardium. Thus, paradoxically, the more uniform the R-R interval becomes and the less chaotic variation it exhibits, the greater the patient’s chances of experiencing sudden cardiac arrest.[120] [A good summary of this phenomenon and its prognostic utility in heart disease is http://circ.ahajournals.org/cgi/reprint/101/1/47] The take home messages here are that the data are only as good as the theory and technology for interpreting them, and that a physician’s summary of raw data, no matter his competence, will, in many instances, be incomplete and sometimes inaccurate, or in any event, lacking the context necessary to yield the maximum benefit for the cryonics patient.
The following case illustrates not only the importance of gathering and evaluating the patient’s medical records, but also the importance of being able to meaningfully interpret the medical history and medical records data in the context of cryonics. I will open this particular analysis by quoting from the case report of Alcor patient A-2063:
“The initial contact for this March 2004 case began at 12:15 (MST), with Hugh Hixon taking the emergency call. A non-member was in the hospital and dying. The gentleman was suffering from terminal cancer and had a subdural hematoma, the result of recent brain surgery. He was suffering from sepsis and pneumonia when Alcor received the call…”
Among the myriad deficiencies in this case report is that virtually no details are given about the patient’s medical Hx, including the patient’s age, sex, weight, race, fat cover, body surface area, underlying medical condition, and so on. However, in this case, because the date and place of medico-legal death are given, it is possible to infer who this patient was, and thus determine that he was a 36 year old, Caucasian male who suffered from acute myelogenous leukemia and whose proximate cause of cardiac arrest was sepsis and thrombocytopenia (inadequate number of circulating blood platelets) following a bone marrow transplant. The thrombocytopenia thus explains the intracranial bleed in the form of a subdural hematoma.
The case report continues as follows (emphasis mine):
“Neuro-perfusion was begun at 09:00, after the head was removed and placed inside the cephalon enclosure. We saw good flow from the left side, but the right jugular showed little venous return. Flow eventually picked up somewhat, but the reason for the obstruction was not determined. Less than twenty minutes later, we noted some swelling of the brain. We attempted to moderate the swelling by slowing perfusion and allowing more time for the cryoprotectant to equilibrate across the hemispheres. The left hemisphere reached terminal concentrations at 15:00, but the right hemisphere had only obtained 59.4% of the concentration needed to vitrify. We continued the neuro perfusion for another five hours before stopping because of toxicity concerns, lowered uptake curves, and staff exhaustion. Final uptake concentration on the left jugular side was 117% of the concentration necessary to vitrify, and the concentration on the right had climbed to 74%. This was the longest neuro-perfusion we’ve ever done.”
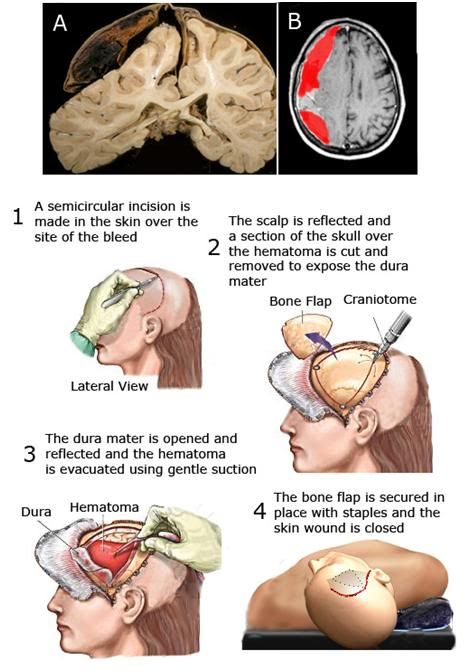 Figure 13: At the top are images of a human brain in the presence of a right subdural hematoma. At left is the brain at autopsy (after fixation and sectioning) showing compression of the right hemisphere and the relative collapse of the right cerebral ventricle and at right is a CT scan with false color (red) showing the appearance of a typical subdural bleed prior to craniotomy to evacuate the accumulated clotted and fluid blood overlying the brain. The rest of the Figure shows the procedure used for surgical management of a subdural hematoma; beginning with an incision in the skin (1) to expose the cranial vault over the area of the hematoma, after which the bone is cut and temporarily removed to expose the dura mater, the fibrous, tough membrane that covers the cerebral hemispheres (2). The dura is then incised and reflected to allow the surgeon to evacuate the hematoma; a process that is usually carried out by gentle suction and saline irrigation of the affected area of the cortical surface (3). Any evident bleeding vessels are clipped and cauterized, the bone flap is replaced and secured in position with metal staples or stainless steel sutures, and the skin flap is reapproximated and (typically) closed with metal staples (4).
Figure 13: At the top are images of a human brain in the presence of a right subdural hematoma. At left is the brain at autopsy (after fixation and sectioning) showing compression of the right hemisphere and the relative collapse of the right cerebral ventricle and at right is a CT scan with false color (red) showing the appearance of a typical subdural bleed prior to craniotomy to evacuate the accumulated clotted and fluid blood overlying the brain. The rest of the Figure shows the procedure used for surgical management of a subdural hematoma; beginning with an incision in the skin (1) to expose the cranial vault over the area of the hematoma, after which the bone is cut and temporarily removed to expose the dura mater, the fibrous, tough membrane that covers the cerebral hemispheres (2). The dura is then incised and reflected to allow the surgeon to evacuate the hematoma; a process that is usually carried out by gentle suction and saline irrigation of the affected area of the cortical surface (3). Any evident bleeding vessels are clipped and cauterized, the bone flap is replaced and secured in position with metal staples or stainless steel sutures, and the skin flap is reapproximated and (typically) closed with metal staples (4).
It is of great importance to note that two physicians were either in consultation, or directly involved in this patient’s care; one of them a Board Certified neurosurgeon. This is both noteworthy and remarkable, when consideration is given to the sentences highlighted in red in the excerpt from the case report, quoted above. We are also told that, “Burr hole drilling was started within five minutes, after shaving the head and disinfecting the scalp, and was completed by 07:29.” While we are not told which side of the patient’s brain the subdural bleed was on, but we are told, “We saw good flow from the left side, but the right jugular showed little venous return. Flow eventually picked up somewhat, but the reason for the obstruction was not determined.” and that likely answers the question for us (the right hemisphere).
A quick glance at Figure 13, above, should also go a long way towards explaining why the “right jugular showed little venous return” at the start of cryoprotective perfusion, and also why cerebral edema developed in this patient. The brain receives ~ 1/3 rd of the resting cardiac output and the internal jugular venous drainage from an isolated cephalon will consist mostly of return from the cerebral hemisphere it drains. A patient presenting for cryopreservation with this medical Hx should be re-imaged during the agonal period and must, before the start of CPA perfusion, have the craniotomy re-opened to ensure there has been no subdural rebleed, accumulation of serosanguineous fluid, or pre-existing cerebral edema that could compromise CPA equilibration in the brain. In this case, none of these things were done.
The case report further notes that, “The left hemisphere reached terminal concentrations at 15:00, but the right hemisphere had only obtained 59.4% of the concentration needed to vitrify. We continued the neuro perfusion for another five hours before stopping because of toxicity concerns, lowered uptake curves, and staff exhaustion. Final uptake concentration on the left jugular side was 117% of the concentration necessary to vitrify, and the concentration on the right had climbed to 74%. This was the longest neuro-perfusion we’ve ever done.” If we do the math, that works out to this patient being exposed to ~ 1-8 M cryoprotectant solution for 11 hours, presumably at a temperature of somewhere between +5 to -3oC, the temperatures Alcor has reportedly used in the past to equilibrate patients with the vitrification solutions B2C and M-22, which it licenses from 21st Century Medicine.
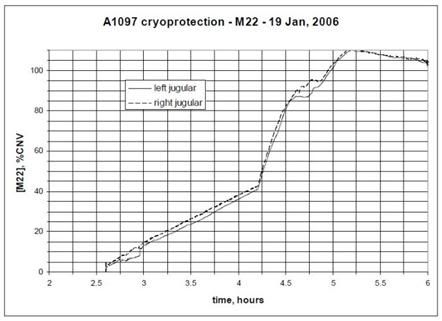
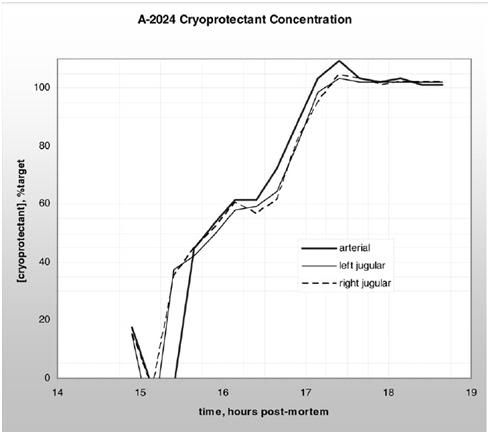 Figure 14: The graph at top shows a 191 minute (3 hr, 11 min) cryoprotective perfusion for the vitrification of Alcor Life Extension Foundation neuropatient A-1097,carried out at the in January of 2006. The vitrification solution employed was M-22, however no patient temperatures during cryoprotectant loading are given in the case report. The graph below shows a 255 minute (4 hr, 15 min) cryoprotective loading of Alcor neuropatient A-2024 with a vitrification solution carried out in April of 2005. The solution used and the temperatures it was perfused at were not disclosed in the case report.
Figure 14: The graph at top shows a 191 minute (3 hr, 11 min) cryoprotective perfusion for the vitrification of Alcor Life Extension Foundation neuropatient A-1097,carried out at the in January of 2006. The vitrification solution employed was M-22, however no patient temperatures during cryoprotectant loading are given in the case report. The graph below shows a 255 minute (4 hr, 15 min) cryoprotective loading of Alcor neuropatient A-2024 with a vitrification solution carried out in April of 2005. The solution used and the temperatures it was perfused at were not disclosed in the case report.
The graphs in Figure 14, above, show typical CPA loading times and curves for two Alcor neuropatients. Frustratingly, neither the graphs or the case reports provide patient temperature data during CPA loading, nor do they provide graphic or tabular data that would allow determination of the mean arterial pressure (MAP) over the course of CPA perfusion. No graphic data for CPA perfusion were given in the case report for A-2036, however, to appreciate the difference in exposure time it is only necessary to multiple 11 hours x 60 minutes to understand that this patient’s CPA perfusion went for 666 minutes; 475 minutes longer than that of patient A-1097 and 411 minutes longer than for that of patient A-2024.
Why did this happen? The answer to that question is present in the patient’s medical Hx, and would have been glaringly evident in the CT and/or MRI scans that no doubt comprised part of the patient’s most recent medical record; principally that he had a large compressive mass of fluid or clotted blood overlying some portion of his right cerebral hemisphere. There are two inevitable and easily foreseeable consequences of this in the context of cryoprotective perfusion. The first is that the clot or fluid mass is not vascularized; it is not permeated by countless capillaries, as is normal brain tissue, with many square centimeters of surface area across which mass transport of CPAs and water can occur. In other words, it is largely ‘dead space’ from which mass exchange can occur only at the surfaces that abut circulated tissues. The second is that such a mass, if it is compressing the brain that underlies it, will reduce or altogether stop flow in the affected cerebral hemisphere. And since cryoprotectant solution is not blood, and does not contain platelets or any other hemostatic agent, any perfusate that leaks from torn or leaking bridging veins on the dura mater, as well as from the pia mater covering the brain, will continue to accumulate in the subdural space, further increasing the intracranial pressure.
In fact, this patient was fortunate that he experienced any cerebral perfusion, because such massive unilateral failure of perfusion of one hemisphere in the presence of a closed cranium is often associated with failure of global brain perfusion, due to elevation of the intracranial pressure to 40–50 mm Hg, which is the range of pressures at which cerebral perfusion, even with severe compensatory hypertension (~250-300 mm Hg), fails.[124, 125] The CPA perfusion pressures used on A-2036 are also not given in the case report, and the patient’s intracranial pressure was either not monitored, or not reported, if it was.
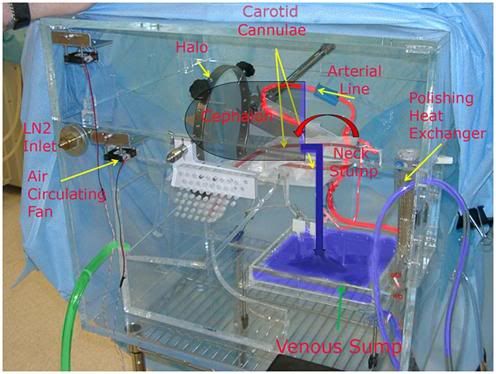 Figure 15: The temperature controlled neuro-cryoprotective perfusion enclosure used by the Alcor Life Extension Foundation. The cephalon is held in position using a standard surgical head fixation halo, with the stump of the neck being positioned over a polycarbonate plastic tray (venous sump, green arrow) that serves as the reservoir to collect venous drainage from the stump of the neck (right angle blue arrow) and return it to the extracorporeal circuit.
Figure 15: The temperature controlled neuro-cryoprotective perfusion enclosure used by the Alcor Life Extension Foundation. The cephalon is held in position using a standard surgical head fixation halo, with the stump of the neck being positioned over a polycarbonate plastic tray (venous sump, green arrow) that serves as the reservoir to collect venous drainage from the stump of the neck (right angle blue arrow) and return it to the extracorporeal circuit.
The proper management of this patient would have been to re-open the craniotomy prior to CPA perfusion and to leave it open for the duration of perfusion.[126-128] This would have eliminated the need for a burr hole (and the associated loss of time experienced in creating it) on the affected side, and it would have allowed for free drainage of any leaking perfusate into the venous sump of the neuroenclosure, where it would have been picked up along with the rest of the venous drainage from the jugulars (and the stump of the patient’s neck), been filtered, and then have been returned to the extracorporeal circuit (Figure 15). In addition, the large opening in the skull would have functioned as a decompressive craniotomy, allowing the ischemia-injured brain to swell during CPA perfusion, without the danger of it raising the intracranial pressure (in the closed cranial vault), thus compromising perfusion to the left hemisphere of the brain, as well.
Why didn’t the “cryonics” physicians, one of them a skilled neurosurgeon, who were attending/consulting for this patient, undertake or suggest this course of action? Many answers to this question are possible, some of them decidedly unflattering, however the most likely reasons are as follows:
1) They do not understand the basic principles and practice of human cryopreservation. They very likely did not give consideration to both why and how CPA perfusion works. Unless the physician understands that effective cryoprotection for vitrification can only be achieved by substituting ~ 60% of the water in the patient’s brain tissue with cryoprotectant molecules (that are on average 1/3rd to ½ the molecular weight of glucose!), and which can only be equilibrated via a patent brain tissue capillary bed, the presence of a compressive mass of non-vascular fluid will have no importance to them.
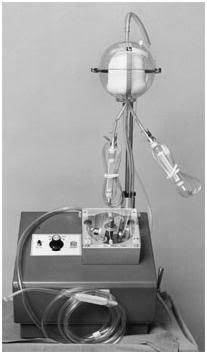 Figure 16: The Bentley Autotransfuser, which made its debut in 1970, allowed emergency medicine physicians and trauma surgeons to recover hemorrhaged blood and rapidly return it to the patient, thus preventing death from exsanguination, and often the need for extensive homologous blood transfusion, as well.
Figure 16: The Bentley Autotransfuser, which made its debut in 1970, allowed emergency medicine physicians and trauma surgeons to recover hemorrhaged blood and rapidly return it to the patient, thus preventing death from exsanguination, and often the need for extensive homologous blood transfusion, as well.
2) In a clinical setting it is essential to secure hemostasis in a surgical wound – any surgical wound – before concluding surgery. Otherwise, the patient will bleed to death, or the wound will become engorged with a pressurized mass of blood (a hematoma) and will subsequently dehisce, or otherwise fail to heal properly. Conventional neurosurgeons do not have the luxury of leaving an open head wound to bleed freely into a catch basin, whereupon the contents are filtered and returned to the venous circulation. However, patients undergoing cryopreservation do have that option, and so do anticoagulated patients undergoing open heart surgery. In the latter case, the blood seeping from and into the sternotomy wound is collected via cardiotomy suction, filtered, and returned to the patient’s circulation. Taking a leaf from the cardiothoracic surgeons, trauma surgeons adapted the cardiotomy suction system in the 1970s and developed auto-transfusion systems (Figure 16) to allow for rapid recovery, reprocessing and reinfusion of a patient’s own shed blood until hemostasis could be secured.[129, 130]
3) They have not been instructed and mentored to care for the cryonics patient as they would any living human being whose medical care they undertook. It is not a precondition that physicians, or other medical professionals (or even volunteers) involved in delivering care to cryopatients, be card carrying (or tag wearing) cryonicists, but it is essential that they understand and share the values of cryonicists, and that they both understand and agree to provide the same level and quality of care that they render to any of their ‘conventional’ medical patients. This knowledge and consent can only be had by a carefully structured course of learning, which actively selects for those men and women capable of such behavior, and which filters out and rejects those who are not. In this respect, such a program of ‘cryomedical residency’ is no different than any other in medicine, in the professions, or in the trades (where the apprenticeship system is used to the same end).
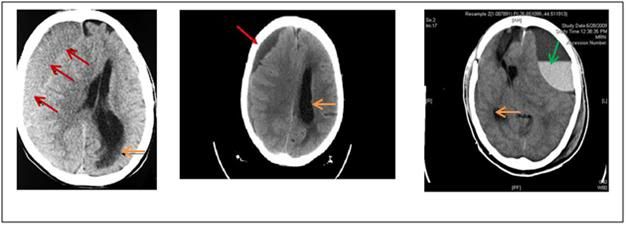 Figure 17: Typical CT images of non-traumatic subdural hematomas (left and center) similar in nature to those frequently seen in spontaneous intracranial bleeds due to thrombocytopenia. The red arrows denote the area of the bleeds, the orange arrows the distortion of the contralateral cerebral ventricles. The green arrow on the image at right shows an air-fluid interface in the left fronto-parietal subdural hematoma cavity with fluid level from burr hole drainage causing mass effect compression the adjacent lateral ventricle (this image was from a case of subdural hematoma secondary to traumatic brain injury). Rebound of the compressed cerebral cortex to normal volume following craniotomy may take several days, providing the patient survives.
Figure 17: Typical CT images of non-traumatic subdural hematomas (left and center) similar in nature to those frequently seen in spontaneous intracranial bleeds due to thrombocytopenia. The red arrows denote the area of the bleeds, the orange arrows the distortion of the contralateral cerebral ventricles. The green arrow on the image at right shows an air-fluid interface in the left fronto-parietal subdural hematoma cavity with fluid level from burr hole drainage causing mass effect compression the adjacent lateral ventricle (this image was from a case of subdural hematoma secondary to traumatic brain injury). Rebound of the compressed cerebral cortex to normal volume following craniotomy may take several days, providing the patient survives.
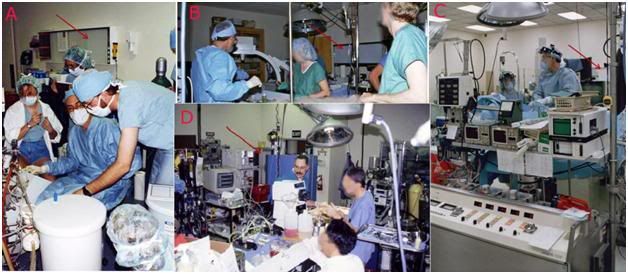 Figure 18: The operating rooms of every cryonics facility the author has had responsibility for over the course of his career all featured an x-ray view box (red arrows) to allow for intra-operative viewing and evaluation of diagnostic electromagnetic patient images. The operating rooms of all currently operational cryonics facilities lack this feature; this has been the case since they were first put into operation. Alcor Foundation, 1989 (A), BioPreservation/PW Biomedical, 1992 (B), BioPreservation, 1993 (C), BioPreservation/21st Century Medicine, 1999 (D).
Figure 18: The operating rooms of every cryonics facility the author has had responsibility for over the course of his career all featured an x-ray view box (red arrows) to allow for intra-operative viewing and evaluation of diagnostic electromagnetic patient images. The operating rooms of all currently operational cryonics facilities lack this feature; this has been the case since they were first put into operation. Alcor Foundation, 1989 (A), BioPreservation/PW Biomedical, 1992 (B), BioPreservation, 1993 (C), BioPreservation/21st Century Medicine, 1999 (D).
The bottom line is that patient A-2036 suffered ~5-7 hours of unnecessary and undoubtedly injurious cryoprotective perfusion (and the associated additional cold ischemia) when a procedure as simple as removing freshly placed staples, and opening an already created surgical wound, would have very likely reduced or eliminated the problem. This happened primarily because of a lack of the ability to meaningfully interpret the patient’s medical history and current medical condition, and possibly to a lesser extent, because the patient’s medical records, and the medical images that were no doubt present in them, were not reviewed at any point prior to undertaking the patient’s cryonics care. It would be hard to imagine that any thoughtful consideration of an image, such as any one of those shown in Figure 17, above, undertaken in the operating room where CPA perfusion was to take place (before the procedure began) on an inexpensive x-ray viewing box (Figure 18) would not have lead to at least some consideration (and thus understanding of the likely consequences of) lesions of this size and location by anyone with any material degree of medical sophistication, coupled with the barest knowledge of the mechanics of cryoprotective perfusion.
Example 3: Pre-Cryopreservation Disease Often Critically Alters Physiology
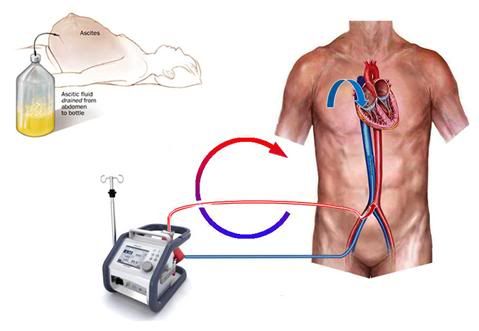 Figure 19: Femoral-femoral bypass in cryopatients is typically carried out by placing a short cannula in the femoral artery, and advancing a long, large diameter cannula through the femoral vein, and into the inferior vena cava (IVC). If the intra-abdominal pressure is elevated due to ascites or edema of the visceral organs (abdominal compartment syndrome), it becomes impossible to obtain adequate venous return, because of compression of the IVC. In the case of ascites, the abdomen may be decompressed by the simple expedient of draining it via a stab wound and a fenestrated tube, as shown in the drawing at the upper left.
Figure 19: Femoral-femoral bypass in cryopatients is typically carried out by placing a short cannula in the femoral artery, and advancing a long, large diameter cannula through the femoral vein, and into the inferior vena cava (IVC). If the intra-abdominal pressure is elevated due to ascites or edema of the visceral organs (abdominal compartment syndrome), it becomes impossible to obtain adequate venous return, because of compression of the IVC. In the case of ascites, the abdomen may be decompressed by the simple expedient of draining it via a stab wound and a fenestrated tube, as shown in the drawing at the upper left.
When blood washout and extracorporeal support are performed in the field, it is necessary to access the circulatory system by cannulating the femoral artery and vein in the groin. When cardiopulmonary bypass (CPB) is carried out in this fashion, the blood flows through the blood vessels in a retrograde fashion – in other words, in the opposite direction from which it normally flows.
Because the blood being pumped from the circuit into the patient is being pumped under pressure into the femoral artery, a short cannula of modest diameter may be used. However, the venous blood, flowing from the body and into the bypass circuit, is flowing at very low pressure, typically at 5-10 mm Hg, and its flow into the circuit reservoir is due to gravity.
As a result, a larger diameter cannula which is much longer, must be used. Ideally, we would like to position the tip of that cannula at the level of the right heart (Figure 12), where the blue arrow is on the schematic in Figure 19, above.[131, 132] However, that is usually not possible to do in the field without x-ray (fluoroscopic) or ultrasound imaging. Thus, the cannula tip is usually in the inferior vena cava (IVC), somewhere below the level of the diaphragm, where the white arrow is pointing in Figure 19.[133] This barely allows for enough venous blood flow out of the patient – even under the best conditions.[134, 135]
If the patient has a large volume of fluid in his abdomen (a condition called ascites), has pre-existing edema of the abdominal viscera, or is very obese, what happens is that the pressure from the excess fluid, fat, or edematous bowel compresses the thin, flexible and essentially unpressurized walls of the vena cava, and prevents adequate venous return.
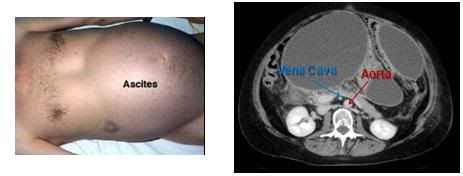 Figure 20: Shown at left above is the fluid distended abdomen of a patient with severe ascites secondary to end-stage liver failure. The protein-rich ascitic fluid ‘weeps’ from the serosa of the liver and accumulates in the abdomen where it compresses the abdominal viscera and can impede venous return from the lower extremities. At right is a CT scan with contrast showing the enormous degree of caval compression that can occur in ascities. The aorta is clearly visible (red arrow), but the IVC, which is normally twice the diameter of the aorta, appears as a small white dot, compressed as it is by the large volume of intra-abdominal fluid (blue arrow).
Figure 20: Shown at left above is the fluid distended abdomen of a patient with severe ascites secondary to end-stage liver failure. The protein-rich ascitic fluid ‘weeps’ from the serosa of the liver and accumulates in the abdomen where it compresses the abdominal viscera and can impede venous return from the lower extremities. At right is a CT scan with contrast showing the enormous degree of caval compression that can occur in ascities. The aorta is clearly visible (red arrow), but the IVC, which is normally twice the diameter of the aorta, appears as a small white dot, compressed as it is by the large volume of intra-abdominal fluid (blue arrow).
The MRI at right in Figure 20, above, shows a typical ascitic abdomen in cross section. Contrast media has been given intravenously so that the blood vessels show up distinctly. The aorta is clearly visible, but the IVC, which is normally twice the diameter of the aorta, appears as a small white dot, compressed as it is by the large volume of intra-abdominal fluid.
Ascites is not uncommon in cryonics patients, since it occurs in cases of liver failure, cancer which has invaded the liver, congestive heart failure, cirrhosis, ovarian cancer and a number of other conditions. If a cryopatient presents with ascites, one of two things must be done before femoral-femoral CPB is undertaken. The ascites may be drained by the simple expedient of making a stab wound through the body wall and placing a drainage tube in the peritoneal cavity, or an alternative venous drainage site must be selected, such as the internal jugular vein.
Failure to do one or the other of these things will result in either no venous return, or inadequate venous return. In the latter case, the effect will be the very rapid development of massive systemic and cerebral edema due to the increased pressure in the venous circulation.
I would be remiss if I did not disclose that the patient whose case I am about to discuss was a lifelong friend, colleague, and personal and professional mentor to me: Curtis Henderson. My feelings for him may be summed up quite simply by saying that I loved him more than my parents, and more than most other men and women who have touched my life. He was also a man who did much to advance to cryonics; and who suffered mightily as a consequence. He deserved the best cryonics care possible. Having disclosed these caveats, I will endeavor to keep my analysis of this case objective (and as dispassionate as possible). I selected this case for review because it represents the most recent of what has been a series of repeated errors in managing ascites in the setting of cryopatient perfusion that now extends back well over a decade.
Curtis Henderson’s Transport case report (Cryonics Institute Member 165, Patient 95
Date: June 25, 2009) may be accessed at: http://www.suspendedinc.com/cases/Stabilization%20and%20Transport%20Case%20Report%20CI95.pdf
Under the heading “CANNULATION” the following narrative is present:
“The vein was 4-5mm in diameter, dark, thin walled, and fragile. Blood flowed freely from it during cannulation. It was ultimately cannulated with a 15 Fr venous cannula inserted approximately 22cm, after attempts to place larger 21 Fr and 19 Fr were unsuccessful.”
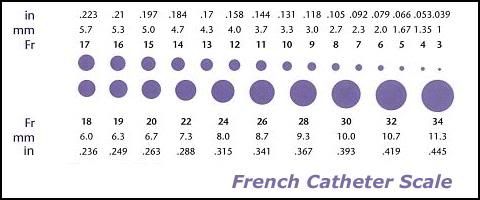 Figure 21: The French (Fr) scale of catheter measurement. Even with the advent of ultra-thin-walled flat-wire femoral venous return cannulae, 21 Fr is the minimum size required to achieve adequate venous return in a 70 kg man under low flow conditions. The combined superior and inferior vena cava diameters, through which blood normally returns to the heart, are on the order 40-60 mm. Flow through tubes (cylinders) is not a linear function of tubing diameter, but rather increases or decreases as a function of the fourth power the radius of the tube (for the same liquid under the same conditions of temperature and pressure).
Figure 21: The French (Fr) scale of catheter measurement. Even with the advent of ultra-thin-walled flat-wire femoral venous return cannulae, 21 Fr is the minimum size required to achieve adequate venous return in a 70 kg man under low flow conditions. The combined superior and inferior vena cava diameters, through which blood normally returns to the heart, are on the order 40-60 mm. Flow through tubes (cylinders) is not a linear function of tubing diameter, but rather increases or decreases as a function of the fourth power the radius of the tube (for the same liquid under the same conditions of temperature and pressure).
Even without having been present, or seeing any photo documentation, it is possible to state with near certainty that the vein cannulated, as described in the text above, was not the femoral vein. The normal femoral vein in an adult human is never 4-5 mm in diameter, but rather is 2 to 3 times that diameter, and will accommodate venous return catheters in the size range of 22 to 32 Fr (see Figure 21). One of the reasons that gross anatomy instruction has historically been accompanied by the dissection of multiple human cadavers, is that knowledge of both the topographical and internal structural elements of the body is essential to being able to perform medical and surgical procedures competently, such as femoral cut-downs; as well as to diagnose disease. While two students are typically assigned to a single human cadaver, the gross anatomy class as whole will typically be dissecting 12 to 15 cadavers, and this allows for the students to appreciate the considerable individual variation in human anatomy.
Following completion of gross anatomy and a medical education, the physician who wishes to become a surgeon, or to perform specific surgical procedures in the conduct of his practice of medicine, undertakes a residency, or assists with the procedure he wishes to master, until such time as both he and his instructor are satisfied that he has achieved a sufficient degree of competence to operate solo. This is a fairly long process with no shortcuts: there are no textbook autodidacts in the art of surgery. Historically, physician-surgeons have been unwilling to participate in human cryopreservation cases, even with the offer of substantial financial inducements. What this has meant in practice is that vascular cannulation of cryopatients has been carried out by morticians. This state of affairs was reasonably satisfactory until the advent of immediate and continuous post cardiac arrest CPS, which introduced a pressurized circulatory system into the equation and all of the complications attendant thereto, such as obstruction of the surgical field by blood from cut, bleeding tissues, frank hemorrhage, and occasionally, widespread contamination of the operator and the mortuary preparation (embalming) room with blood when a pressurized, large caliber artery was incised, absent proper precautions to control bleeding or deal with the ensuing contamination (i.e. heavy duty face shields, Tyvek or other impermeable clothing, etc.).
Beginning in the late 1970s, two non-physician-surgeon cryonics professionals, Jerry Leaf and myself, made the decision to become proficient at the modest repertoire of surgical procedures then perceived to be needed in the practice of human cryopreservation. We were fortunate in that both of us had had extensive experience in animal research (wherein we had been trained to carry out most of the procedures we sought to master) on dogs, pigs and other mammals. Additionally, both of us had undertaken independent didactic study of both human and veterinary anatomy. Still, we found it necessary to obtain access to human cadavers, and to observe the procedures we were undertaking to learn being performed by skilled surgeons on living human patients. No claim is made here, express or implied, that we ever approached the level of skill of a competent (human) general, cardiothoracic or vascular surgeon.
Our principal deficiencies were lack of speed (competent surgeons operate both with great speed and great precision) and lack of the deep base of experience that facilitates rapid identification and resolution of anomalies, or unexpected problems. These may seem formidable handicaps, and indeed they were. However, it is uniquely in the nature of cryonics that the most complex and complication ridden parts of the operative procedures we employ present themselves when the patient is in profound, or ultraprofound hypothermia, which means we have the advantage of some ‘breathing room’ when something goes wrong. A femoral cut-down and cannulation for CPB is much less complicated than surgery, instrumentation/monitoring, and cannula placement for CPB using the aortic root and right atrium approach. Another fortunate circumstance is that femoral-femoral CPB in the dog very closely approximates the experience with humans in terms of surgical complications and difficulties, such as cannula placement, bleeding, and even the basic anatomy of the femoral vessels. Thus, extensive survival fem-fem CPB experience with dogs, coupled with (mortuary) practice on human cadavers, provided us with the skill, if not the speed, to perform femoral cannulation for CPB with a high degree of confidence and a low incidence of complications.
The case report continues:
Nearly over the top of the vein, was the artery with multiple feeder vessels between the two. The artery was 6-7mm in diameter, light colored, rubbery and heavy walled. Bright red blood flowed freely from it during cannulation. It was cannulated with a 17 Fr arterial cannula inserted approximately 12cm after attempts with a 19 Fr cannula were unsuccessful.
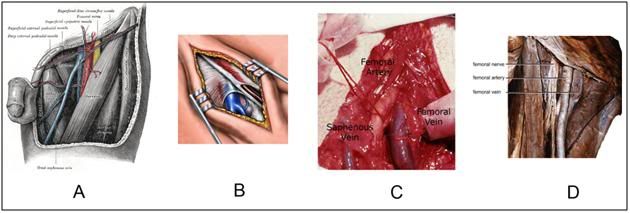
Figure 22: The anatomy of the femoral vessels as shown in Gray’s Anatomy (A), an idealized artist’s rendering of exposure of the femoral vein for cannulation (B), as seen in a human cryonics patient undergoing CPS in the 1970s (mortician’s cut-down) and as seen in a cadaver prepared for dissection by medical students (D). The femoral artery is quite superficial and the femoral vein is fairly deep. It is easily possible for an inexperienced operator to mistake the saphenous vein (see A & C) for the femoral vein and attempts its cannulation. Only knowledge of the anatomy of the femoral triangle, and prior experience with and observation of the femoral vein, can ensure against this mistake. The experienced operator knows the appearance, location, and above all, the large size of the femoral vein and will, if necessary, increase the depth and scope of dissection until it is identified and raised.
The keys to successful dissection, identification and cannulation of the femoral vessels are a knowledge of the anatomy of the femoral triangle (and its all too common vascular variations) and prior visual and tactile experience with the procedure. As can be seen in Figure 22, above, the femoral vein is a large caliber vessel with comparatively thick walls (for a vein), and it is easily identified, even when collapsed and denuded of color, as is evident in 22-D, above.
 Figure 23: If the operator’s thumb is placed on the anterior superior iliac spine and the third finger is positioned atop the pubic tubercle, the index finger will usually be positioned approximately over the femoral neurovascular bundle, which contains the femoral artery and vein (left, above). At right, above, is an anterior view of the right thigh: V=femoral vein; A=femoral artery, N=femoral nerve, 1=adductor longus, 2=adductor brevis, 3=pectineus, 4=iliopsoas and 5=sartorius muscles. If a skin incision is made paralell to the midline as illustrated by the purple line in the figure at left, above, at the midinguinal point, the femoral vein will typically be found a few millimeters, to half a centimeter medial to the femoral artery. The use of these topographical landmarks and knowledge of the underlying muscular anatomy, is essential in performing femoral cut-downs on cryopatients, because the femoral pulse may be absent or not palpable, even when closed chest CPS is ongoing.
Figure 23: If the operator’s thumb is placed on the anterior superior iliac spine and the third finger is positioned atop the pubic tubercle, the index finger will usually be positioned approximately over the femoral neurovascular bundle, which contains the femoral artery and vein (left, above). At right, above, is an anterior view of the right thigh: V=femoral vein; A=femoral artery, N=femoral nerve, 1=adductor longus, 2=adductor brevis, 3=pectineus, 4=iliopsoas and 5=sartorius muscles. If a skin incision is made paralell to the midline as illustrated by the purple line in the figure at left, above, at the midinguinal point, the femoral vein will typically be found a few millimeters, to half a centimeter medial to the femoral artery. The use of these topographical landmarks and knowledge of the underlying muscular anatomy, is essential in performing femoral cut-downs on cryopatients, because the femoral pulse may be absent or not palpable, even when closed chest CPS is ongoing.
To facilitate rapid and accurate location of the correct anatomical area to dissect, a simple procedure may be used to make a first approximation for the skin incision, as shown in Figure 23, above.
The case report continues:
The cannulae were connected to the extracorporeal bypass circuit on the Stockert SCPC minibypass system that had been primed with MHP2 organ preservation solution and cooled by the perfusionist. No venous drainage was observed. No bubbles or air locks were visible in the circuit. The perfusionist applied mild suction and the AutoPulse was re-started to assist with drainage. Still, no venous return could be seen. The venous cannula was slowly backed out while applying suction and automated chest compressions but no return was visible.
Nasopharyngeal temperature was 15C and rectal was 25.6C. A call was made to CI to determine additional site options for cannulation. A jugular cannulation would not interfere with cryoprotection procedures. The patient’s head was repositioned to the contralateral side and the neck swabbed and prepped for external jugular vein cutdown. The AutoPulse was started to aid location of jugular vein.
Pressure to the platysma muscle did not create any obvious jugular pooling. Identification of the external jugular was then made using the mid-point between the angle of the mandible and the top of the clavicle. Using a number 10 scalpel blade, a 3cm incision was made and blunt dissection used to clear the tissue. The jugular was not immediately visible.
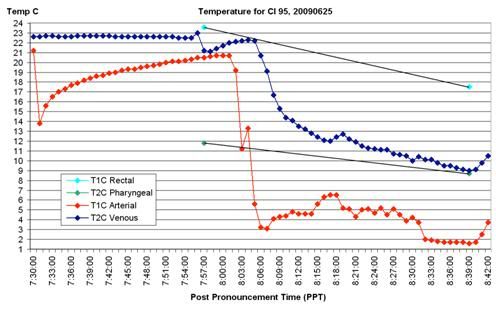 Figure 24: The graphic presentation of temperature data for CI Patient 95 documents the lack of venous return as an almost certain consequence of abdominal compartment syndrome, secondary to ascites. The arterial temperature shows a sharp drop as CPB is initiated, however the patient’s (circuit) venous temperature remains constant and does not begin to decline below ambient temperature until CPB is re-initiated an hour after the first attempt was made. The reader is encouraged to study all the data on this graph, and to carefully read the case report in its entirety, because I will be returning to this case and this data in a future installment of this article to make a point about another serious deficiency in the CPS of this patient. More precisely, I will be asking you, the readers, to identify this problem and ascertain its cause.
Figure 24: The graphic presentation of temperature data for CI Patient 95 documents the lack of venous return as an almost certain consequence of abdominal compartment syndrome, secondary to ascites. The arterial temperature shows a sharp drop as CPB is initiated, however the patient’s (circuit) venous temperature remains constant and does not begin to decline below ambient temperature until CPB is re-initiated an hour after the first attempt was made. The reader is encouraged to study all the data on this graph, and to carefully read the case report in its entirety, because I will be returning to this case and this data in a future installment of this article to make a point about another serious deficiency in the CPS of this patient. More precisely, I will be asking you, the readers, to identify this problem and ascertain its cause.
As the case narrative continues, problems encountered and overcome 20+ years ago in cryonics in-field operations (and overcome long before, in convention surgery) surface and are noted, but are not remarked upon:
“Opening an 8 cm incision and using an aneurism hook for dissection, his field quickly filled with bright red blood. He located the femorals but in separating them, he accidentally cut the artery and multiple feeders between the artery and vein. These vessels were individually ligated and the wound packed while the funeral director enlarged the jugular incision that had been opened earlier and isolated the jugular. A 17Fr venous cannula was inserted into the jugular vein approximately 30cm and connected to the venous perfusion line. Mild suction was applied.
Venous drainage was observed.”
Morticians are unprepared to perform vascular surgery on cryonics patients who are undergoing CPS, or who have been subjected to CPS with heparinization and prompt external cooling (usually with bulk ice present and compressing the tissues). The rough handling of the tissues and vessels that they are accustomed to using with impunity on corpses, quickly degenerates into catastrophes for which they have no ready solution. Additionally, the absence of high capacity electrically powered suction (‘hot’ suction) leaves the operator unable to visualize the surgical field. Attempts to blot the wound with absorbent material merely result in replacing one visualization obstruction with another; since the instant the sponge is removed from the wound, it refills with opaque blood. These are foolish and inexcusable errors only because they have been made before, are documented in the cryonics case literature,[136] and have solutions that are easily learned and implemented – but only if those attempting to practice cryonics as medicine take the time, and exercise the diligence required to learn them.
The problem of ascites in the context of femoral-femoral CPB has occurred at least five times in cryonics cases that I know of, and in three of those five cases, it happened to personnel who had experienced the same problem before. And yet, the problem was not addressed and the same rote procedure was followed, despite the fact that problems were evident. I will say that in two cases where there was no venous return they did eventually stop perfusion because they realized that ‘something was wrong.’ In this case, the presence of a skilled perfusionist undoubtedly prevented the iatrogenic disaster of more than a very brief period of injection of arterial perfusate in absence of sufficient (or any) venous return. There are myriad other problems and deficiencies in this case (as adjudged from the report), all of which were and are avoidable with the hard-won, existing base of knowledge, wisdom and professionalism that has been generated since the scientific practice of cryonics was begun by Fred and Linda Chamberlain, Greg Fahy and me over 30 years ago.
Nevertheless, the real solutions to the problems discussed here are not easy, because they demand the acquisition of professionalism, knowledge, and skill in the context of cryonics as medicine. I personally believe that Jerry Leaf and I came very close to doing that in the decade between 1981 and 1991. But we failed. Why we failed will be discussed at a later time. Suffice it to say that the problem of maintaining professionalism is a nettlesome one in medicine, engineering and other demanding disciples that are vastly more developed than cryonics is today, and there will be no quick fixes. In cryonics, where almost all the feedback we get from our patients must be artificially generated, the problem will be much more difficult to solve. However, no progress will be made absent understanding and acknowledgement of the need to acquire and interpret the patient’s medical records in a timely fashion, whilst being informed by both medical and cryonics knowledge and professionalism.
End of Part 4
References
119. Hippocrates: Of The Epidemics: http://classics.mit.edu/Hippocrates/epidemics.1.i.html; 400 BCE.
120. Huikuri H, Mäkikallio, TH, Peng, CK, Goldberger, AL, Hintze, U, Møller, M.: Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 2000, 4(101(1)):47-53.
121. Laitio T, Huikuri, HV, Kentala, ES, Mäkikallio, TH, Jalonen, JR, Helenius, H, Sariola-Heinonen, K, Yli-Mäyry, S, Scheinin, H.: Correlation properties and complexity of perioperative RR-interval dynamics in coronary artery bypass surgery patients. . Anesthesiology 2000, 93(1):69-80.
122. Pikkujämsä S, Mäkikallio, TH, Sourander, LB, Räihä, IJ, Puukka, P, Skyttä, J, Peng, CK, Goldberger AL, Huikuri, HV.: Cardiac interbeat interval dynamics from childhood to senescence : comparison of conventional and new measures based on fractals and chaos theory. Circulation 1999, 27(100(4)):393-399.
123. Schmitt D, Ivanov, PCh.: Fractal scale-invariant and nonlinear properties of cardiac dynamics remain stable with advanced age: a new mechanistic picture of cardiac control in healthy elderly. Am J Physiol Regul Integr Comp Physiol 2007, 293(5):R1923-1937.
124. Verweij BH, Muizelaar JP, Vinas FC: Hyperacute measurement of intracranial pressure, cerebral perfusion pressure, jugular venous oxygen saturation, and laser Doppler flowmetry, before and during removal of traumatic acute subdural hematoma. J Neurosurg 2001, 95(4):569-572.
125. Marx JA e (ed.): Head injury (Chapter 38). Philadelphia: Mosby Elsevier; 2009.
126. Guresir E, Schuss P, Vatter H, Raabe A, Seifert V, Beck J: Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg Focus 2009, 26(6):E4.
127. Daboussi A, Minville V, Leclerc-Foucras S, Geeraerts T, Esquerre JP, Payoux P, Fourcade O: Cerebral hemodynamic changes in severe head injury patients undergoing decompressive craniectomy. J Neurosurg Anesthesiol 2009, 21(4):339-345.
128. Eberle BM, Schnuriger B, Inaba K, Gruen JP, Demetriades D, Belzberg H: Decompressive craniectomy: surgical control of traumatic intracranial hypertension may improve outcome. Injury, 41(7):934-938.
129. Klebanoff G: Early Clinical experience with a disposable unit for intraoperative salvage and reinfusion of blood loss intraoperative autotransfusion). Am J Surg 1970, 120:718-722.
130. Symbas PN: Extraoperative autotransfusion from hemothorax. Surgery 1978, 1078 (84):722.
131. Phillips SJ, Ballentine B, Slonine D, Hall J, Vandehaar J, Kongtahworn C, Zeff RH, Skinner JR, Reckmo K, Gray D: Percutaneous initiation of cardiopulmonary bypass. Ann Thorac Surg 1983, 36(2):223-225.
132. von Segesser LK, Kalejs M, Ferrari E, Bommeli S, Maunz O, Horisberger J, Tozzi P: Superior flow for bridge to life with self-expanding venous cannulas. Eur J Cardiothorac Surg 2009, 36(4):665-669.
133. Kirkeby-Garstad I, Tromsdal A, Sellevold OF, Bjorngaard M, Bjella LK, Berg EM, Karevold A, Haaverstad R, Wahba A, Tjomsland O et al: Guiding surgical cannulation of the inferior vena cava with transesophageal echocardiography. Anesth Analg 2003, 96(5):1288-1293, table of contents.
134. Colangelo N, Torracca L, Lapenna E, Moriggia S, Crescenzi G, Alfieri O: Vacuum-assisted venous drainage in extrathoracic cardiopulmonary bypass management during minimally invasive cardiac surgery. Perfusion 2006, 21(6):361-365.
135. Abdel-Sayed S, Favre J, Horisberger J, Taub S, Hayoz D, von Segesser LK: New bench test for venous cannula performance assessment. Perfusion 2007, 22(6):411-416.
136. Darwin, M:Neuropreservation of Alcor Patient A-1068. Cryonics 1985, 6(4):10-19: http://www.alcor.org/Library/html/casereport8504.html

>In the past two decades I have had the frustrating experience of having no fewer than four Officers, Directors or Presidents of currently extant cryonics organizations dismiss the need to gather and thoroughly analyze the medical records and medical history of cryopatients.
I appreciate your efforts to re-inject some reality into the cryonics movement, Mike. The resistance to gathering and studying members’ medical records seems especially odd, considering that some prominent cryonicists advocate the collecting and digitzing of all sorts of trivial information about themselves to create “mind files,” “life blogs” or whatever trendy name they’ve given to this fad these days. The negligence of something as basic as medical records just reinforces the perception of cryonics as an unserious exercise.
Thanks, Mark. I appreciate your appreciation. — Mike Darwin
Re: >Two cryonics organizations Presidents have informed me that these materials are not only hard to gather, but in addition, are of little or no use to the patient, since future medical diagnostic and treatment modalities will render such information superfluous at best, and misleading at worst.
This illustrates the pernicious effects of a certain kind of “futurology” on cryonics. Apparently many long-time cryonicists don’t see how much of “the future” has become our recent past. The real 21st Century in many ways doesn’t much resemble the sales job we received about it in the mid to latter 20th Century; yet many cryonicists apparently still live mentally in 1990 or so, and talk about an imminent “nanoassembler breakthrough” and similar fantasies as rationalizations for not improving cryonics in the here-and-now.
so, mike, is there an heir apparent in cryonics, someone with your level of knowledge in the area of field cryopreservation? Or does that person exist, but does publicly display his/her knowledge?
How do the people alcor currently use compare to you?
are you willing to teach someone?
Good questions, but you’ve got the grammatical number wrong. It’s not “heir apparent,” but rather, heirs apparent” – and in this case, that makes all the difference in the world. First, the simple and hopefully obvious point; one person isn’t going to be sufficient on purely practical grounds. First, sooner rather than later, that individual will find he is terminally ill and then (if lucky) cryopreserved. Then what?
Jerry Leaf and I used to joke with each other that one of the most compelling reasons we decided to work with each other was that we each had this recurring nightmare where there is a a cryonics emergency. The medical personnel in the emergency department (ED) call the cryonics organization’s answering service, the answering service in turn pages the cryonics technician on-call, and then… the beeper goes off in the ED; and it is on the belt of the “patient” they are calling about, namely, Jerry, or me! Well, as it worked out, that call came in around midnight as I sat in the living room with my feet up on the coffee table watching the Tonight Show with Johnny Carson. It was Hugh Hixon, not an answering service (in those days Alcor personnel actually answered the phone all the time, every time, 24/7/365) and he informed me that Jerry had just been pronounced at Downey Community Hospital.
Unlucky Jerry, or so it would seem, but who knows, maybe he was the luckier one, after all. Time will tell. So, on practical grounds, there isn’t going to be any one person.
It’s also important to understand that no one man can be “it.” Even groups of men, working g together in professions, or in the sciences, can barely manage, and in professions such as the law and medicine, the results are uneven, at best. Contrast this with, say, aviation or dentistry, where the results are much more consistent and salutary – indeed , in aviation, the results are so uniformly good, it is almost unbelievable!
What may not be as obvious, but what is vastly more important, is that it was never just one man, and it never will be. Hell, I won’t hesitate for a moment in saying that, right now, I’m “Who’s on first.” That’s how the dice rolled, and how a lot of events and decisions, some made thoughtfully, some made for us, and some made stupidly by us (or in this case mostly by me) have turned out to created a situation where there’s now apparently just a lone survivor from that era who has the inclination to change, or to alter the situation that now exists. Sure, there are people like Brian Wowk, who have the breadth of knowledge, skill, and the inherent professionalism to change this situation – but they either cannot, or will not. And, in point of fact, I am currently in exactly the same position. With the exception of a few people in Russia, there isn’t a single person in a position of influence or power in institutional cryonics today, who would make even the slightest effort to empower me to change this situation.
The reasons for this vary, but the result is the same. If you hose away all the bullshit, the core reason for this is that they just don’t see the problem as being that serious or that urgent. Certainly, a fair number of people know that things are really bad, and I predict you will see few, if any specific public disagreements with my analyses here. There are confidential internal documents that would make even some of the thicker rank and file cryonicists’ hair stand on end; if they read them. The Officers and Directors of all the extant US cryonics organizations (and of KrioRus) know what the score is in terms of patient care. And many of them genuinely care. That’s not the issue. [And, BTW, it has taken me a ridiculously long time to understand this.]
No, the problem is far more complex. One one level, it is analogous to what you so frequently see when something goes catastrophically wrong, and everyone inside the extant institutions drop the ball. Consider TEPCO, and the unfolding disaster at the Fukushima Daiichi nuclear power plant. First there was denial (just as there was at Chernobyl), then there was disbelief (it just can’t be that bad!) and next, there is the mistaken belief that the problem can be dealt with discreetly and, above all internally and with the institution’s existing resource base. After that, things can unfold in a variety of ways.
If there is no feedback that FORCES action (i.e., you go bankrupt, the police incarcerate you…) then, after a period of years, dysfunctional becomes the accepted norm, and what’s more, it becomes the vigorously defended norm. People get angry, really angry, if the situation is criticized, and they will say, with great conviction of belief, “this is the best we can do with what we’ve got, and what you are doing is not constructive, in fact you are attacking cryonics and attacking our cryonics organization(s).
Some organizations may simply say, “Well, it’s sad, but that’s just the way it is. Guys like you and Jerry were an anomaly, and we need to realize that, and adjust our expectations accordingly.” Finally, if the failure mode goes on long enough, say 20 years or so, then you have a truly monstrous problem to deal with, because 20 years is a human generation, and that means that virtually all of the people doing cryonics will have known no other reality than that of what is, in fact, a failure mode.
These people, quite understandably, become enraged if they are told, no matter how delicately, that they don’t know what they are doing and that they have spent a great deal of time and effort (a lifetime, in fact!) learning to do cryonics in anything less than in a very competent fashion. And if they get a whiff of the idea that their practice of cryonics is, in fact, incompetent, or damaging and destructive, well then, you’ve got a group of very certain and very angry people who, at best, want to quietly suffocate you, and at worst, want to beat the crap out of you and then slowly suffocate you. Meanwhile, those who really are in the know, will continue to hold onto to the belief that with more time, more money, or more store-bought “medical expertise,” they will indeed solve the problem.
At ACS, there is more or less quiet acceptance, and this has been the case since around 2001. At CI, there is anger and the certain belief that they are doing the very best that can be done, given their resources, circumstances, and the financial and logistic situations of their patients. In Alcor’s case, the response has been more complex and, unintentionally, pretty horrific. With the exception of Joe Waynick and Tanya Jones, all the people the Alcor Board has brought in to try to “fix the problem” have been basically decent people. Some of them, like Dave Shipman, were very nice people who also brought a lot of business and technical know-how to the table. None of them wanted to hurt Alcor or cryonics, and I believe all of them wanted to “right the ship,” so to speak.
Not to put too fine a point on it, these people might as well have been put in chains in the Tower, and thence led to the chopping block. They never had a chance.
One reason for this, leaving technology out of it, is that there is no moral or ethical code in cryonics – none. There really aren’t even any clearly defined values. Let’s take a very practical example: A patient, or the patients are under attack, and you are the cryonics organization CEO, or Director. What are the limits of your responsibility? If the authorities come to you and say that you will either give over the patient(s), or they’ll strip you of your livelihood (i.e., take your medical or law license away, get your employer to discharge you…) is it permissible for you to hand over the patient(s)? What if they threaten your family, and/or other uninvolved, and completely “innocent” people? Is it OK, then? What if they threaten to imprison you, and even put the death penalty on the table, or they offer to spare some patients (those that may matter most to you, personally) if you just given them the one(s) they want? These are not hypothetical questions – they actually happened to me, and to the other Alcor Directors in the opening months of 1987.
Let me frame the question a bit differently in order to show you just how dangerous having no defined values is. Suppose that the state comes to you and says, “We will shut you down unless you give us control over your patients.” What is the right thing to do? What PRINCIPLES or VALUES will you use to guide you in making such a decision? The answer is NONE. We aren’t even “making it up as we go,” because that would imply that we keep a record our actions, and refer to these decisions as precedents. In fact, we don’t even do this!
And this situation isn’t hypothetical either, because when the Cemetery Board came down on CI, CI, and thus ACS, decided to surrender control of their patients to the state. Now, it is the laws and jurists of the state of Michigan that determine the conditions under which a patient can be removed from a cryostat at CI, and be relocated elsewhere, not the CEO or the Board of either CI, or ACS. If you want to understand the practical implications of this, you can go to http://www.bhsj.org/forms/disinterment%20and%20reinterment.pdf and to http://law.onecle.com/michigan/333-health/mcl-333-2853.html and read what you find there. It isn’t pretty.
A consequence of this is that anyone who steps into a position of leadership in cryonics does so with no rudder to guide them, and no set of standards to which they can be held accountable, or conversely, to which they can turn to defend themselves against unreasonable expectations, or worse, unreasonable charges of misconduct. In particular, the man who steps into the Presidency of Alcor is a man who has entered a country with no laws, but which is nevertheless populated by judges and jurors – each of whom will decide his fate on unknown, unknowable, shifting, and all too often completely arbitrary rules. The Directors, who are for all intents and purposes anonymous, never suffer the consequences of this grotesque situation (unless they err by becoming visible in their personal decision making or are otherwise conspicuous), even as they select victim after victim for the rack and the chopping block.
Beyond this meta-problem, there are the issues of specific technical knowledge, craft, and the culture of professionalism – all of which are either missing, or in serious disarray, when it comes to scientific cryonics (i.e., feedback and research driven cryonics) as opposed to “proceduralistic” or “ritualistic” cryonics, where a rote set of actions is carried out, without any meaningful feedback – rather like a blind baker, who also suffers from anosmia and ageusia, who cooks up pastries and cakes year after year, which are taken away with nary a word about their palatability, or lack thereof, ever returning to his ears. As long as the Boards and Officers of cryonics organizations lack nocioception, this kind of nightmarish landscape will continue to be the one that dominates the cryonics event horizon.
In large measure it’s the purpose of Chronosphere to repair cryonics on these very fundamental levels.
Finally, you ask, “are you willing to teach someone?”
The answer to this is, “No, I am not willing to teach some one, but I am working as hard as I can to establish the framework to instruct and mentor, and be instructed and mentored in return, by a group of like-minded people who share my commitment to cryonics, and my ideal of what it once was, and can be again.” The question now becomes are there the men and resources out there to permit that to happen, and do we (all of us) have enough time to make it happen before cryonics teeters off the precipice and crashes and burns – at least for us, here and now?
If the answer to those questions is “yes,” then an institutional framework must be created to facilitate the regeneration and creation of a cryonics that is informed by the scientific method, as well as a rational and fair morality. — Mike Darwin