by Michael Darwin, Jerry Leaf, Hugh L. Hixon
I. Introduction
II. Materials and Methods
III Effects of Glycerolization
IV. Gross Effects of Cooling to and Rewarming From -196°C
I. INTRODUCTION
The immediate goal of human cryopreservation is to use current cryobiological techniques to preserve the brain structures which encode personal identity adequately enough to allow for resuscitation or reconstruction of the individual should molecular nanotechnology be realized (1,2). Aside from two previous isolated efforts (3,4) there has been virtually no systematic effort to examine the fidelity of histological, ultrastructural, or even gross structural preservation of the brain following cryopreservation in either an animal or human model. While there is a substantial amount of indirect and fragmentary evidence in the cryobiological literature documenting varying degrees of structural preservation in a wide range of mammalian tissues (5,6,7), there is little data of direct relevance to cryonics. In particular, the focus of contemporary cryobiology has been on developing cryopreservation techniques for currently transplantable organs, and this has necessarily excluded extensive cryobiological investigation of the brain, the organ of critical importance to human identity and mentation.
The principal objective of this pilot study was to survey the effects of glycerolization, freezing to liquid nitrogen temperature, and rewarming on the physiology, gross structure, histology, and ultrastructure of both the ischemic and non-ischemic adult cats using a preparation protocol similar to the one then in use on human cryopreservation patients. The non-ischemic group was given the designation Feline Glycerol Perfusion (FGP) and the ischemic group was referred to as Feline Ischemic Glycerol Perfusion (FIGP).
The work described in this paper was carried out over a 19-month period from January, 1982 through July, 1983. The perfusate employed in this study was one which was being used in human cryopreservation operations at that time, the composition of which is given in Table I.
The principal cryoprotectant was glycerol.
II. MATERIALS AND METHODS
Pre-perfusion Procedures
Nine adult cats weighing between 3.4 and 6.0 kg were used in this study. The animals were divided evenly into a non-ischemic and a 24-hour mixed warm/cold ischemic group. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Institutes of Health (NIH Publication No. 80-23, revised 1978). Anesthesia in both groups was secured by the intraperitoneal administration of 40 mg/kg of sodium pentobarbital. The animals were then intubated and placed on a pressure-cycled ventilator. The EKG was monitored throughout the procedure until cardiac arrest occurred. Rectal and esophageal temperatures were continuously monitored during perfusion using YSI type 401 thermistor probes.
Following placement of temperature probes, an IV was established in the medial foreleg vein and a drip of Lactated Ringer’s was begun to maintain the patency of the IV and support circulating volume during surgery. Premedication (prior to perfusion) consisted of the IV administration of 1 mg/kg of metubine iodide to inhibit shivering during external and extracorporeal cooling and 420 IU/kg sodium heparin as an anticoagulant. Two 0.77 mm I.D. Argyle Medicut 15″ Sentinel line catheters with Pharmaseal K-69 stopcocks attached to the luer fittings of the catheters were placed in the right femoral artery and vein. The catheters were connected to Gould Model P23Db pressure transducers and arterial and venous pressures were monitored throughout the course of perfusion.
Surgical Protocol
Following placement of the monitoring catheters, the animals were transferred to a tub of crushed ice and positioned for surgery. The chest was shaved and a median sternotomy was performed. The aortic root was cleared of fat and a purse-string suture was placed, through which a 14-gauge Angiocath was introduced. The Angiocath, which served as the arterial perfusion cannula, was snared in place, connected to the extracorporeal circuit and cleared of air. The pericardium was opened and tented to expose the right atrium. A purse-string suture was placed in the apex of the right atrium and a USCI type 1967 16 fr. venous cannula was introduced and snared in place. Back-ties were used on both the arterial and venous cannulae to secure them and prevent accidental dislodgment during the course of perfusion. Placement of cannulae is shown in Figure 1.
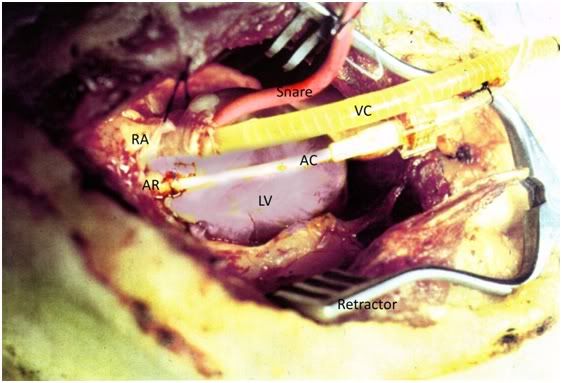
Figure 1: Vascular access for extracorporeal perfusion was via median sternotomy. The arterial cannula consisted of a 14-gauge Angiocath (AC) which was placed in the aortic root (AR) and secured in place with a purse string suture. A USCI type 1967 16 fr. venous cannula (VC) was placed in the right atrium (RA) and snared in place using 0-silk ligature and a length of Red Robinson urinary catheter (snare). The chest wound was kept open using a Weitlander retractor. The left ventricle (LV) was not vented.
Extracorporeal Circuit
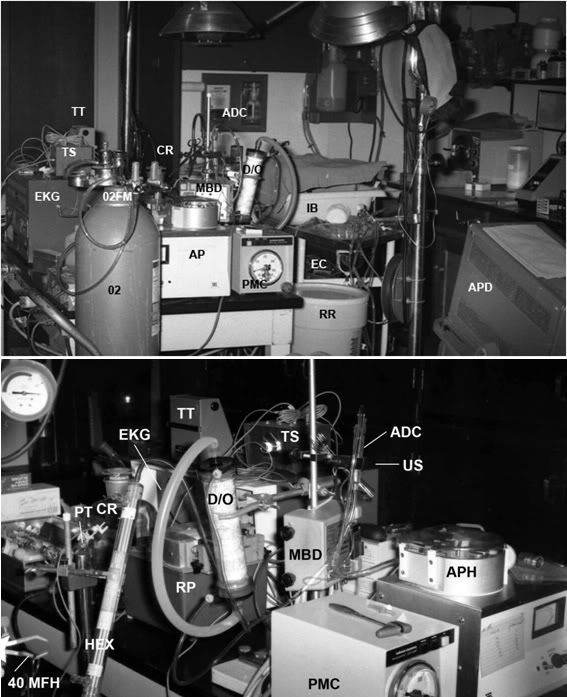
Figure 2: Cryoprotective perfusion apparatus: RR = recirculating reservoir, PMC = arterial pressure monitor and controller, MBD = micro-bubble detector, US = ultrasonic sensor, ADC = arterial drip chamber, D/0 = dialyzer/oxygenator, RP = cryoprotective ramp pump, HEX = arterial heat exchanger, 40 MFH = 40 micron filter holder, PT = arterial pressure transducer, CR = glycerol concentrate reservoir, EKG = electrocardiograph, TT = thermistor thermometer, TS = thermistor switch box, IB = ice bath, EC = electrocautery, APD = arterial pressure display.
The extracorporeal circuit (Figures 2&3) was of composed of 1/4″ and 3/8″ medical grade polyvinyl chloride tubing. The circuit consisted of two sections: a recirculating loop to which the animal was connected and a glycerol addition system. The recirculating system consisted of a 10 liter polyethylene reservoir positioned atop a magnetic stirrer, an arterial (recirculating) roller pump, an Erika HPF-200 hemodialyzer which was used as a hollow fiber oxygenator (8) (or alternatively, a Sci-Med Kolobow membrane oxygenator), a Travenol Miniprime pediatric heat exchanger, and a 40-micron Pall LP 1440 pediatric blood filter. The recirculating reservoir was continuously stirred with a 2″ Teflon-coated magnetic stir bar driven by a Corning PC 353 magnetic stirrer. Temperature was continuously monitored in the arterial line approximately 15.2 cm from the arterial cannula using a Sarns in-line thermistor temperature probe and YSI 42SL remote sensing thermometer. Glycerol concentrate was continuously added to the recirculating system using a Drake-Willock dual raceway hemodialysis pump, while venous perfusate was concurrently withdrawn from the circuit and discarded using a second raceway in the same pump head.
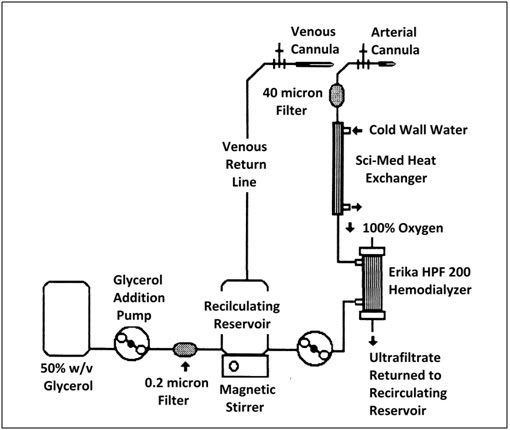
Figure 3: Schematic of cryoprotective perfusion circuit.
Storage and Reuse of the Extracorporeal Circuit
After use the circuit was flushed extensively with filtered tap and distilled water, and then flushed and filled with 3% formaldehyde in distilled water to prevent bacterial overgrowth. Prior to use the circuit was again thoroughly flushed with filtered tap water, and then with filtered distilled water (including both blood and gas sides of the hollow fiber dialyzer; Kolobow oxygenators were not re-used). At the end of the distilled water flush, a test for the presence of residual formaldehyde was performed using Schiff’s Reagent. Prior to loading of the perfusate, the circuit was rinsed with 10 liters of clinical grade normal saline to remove any particulates and prevent osmotic dilution of the base perfusate.
Pall filters and arterial cannula were not re-used. The circuit was replaced after a maximum of three uses.
Preparation of Control Animals
Fixative Perfusion
Two control animals were prepared as per the above. However, the animals were subjected to fixation after induction of anesthesia and placement of cannulae. Fixation was achieved by first perfusing the animals with 500 mL of bicarbonate-buffered Lactated Ringer’s containing 50 g/l hydroxyethyl starch (HES) with an average molecular weight of 400,000 to 500,000 supplied by McGaw Pharmaceuticals of Irvine, Ca (pH adjusted to 7.4) to displace blood and facilitate good distribution of fixative, followed immediately by perfusion of 1 liter of modified Karnovsky’s fixative (Composition given in Table I). Buffered Ringers-HES perfusate and Karnovsky’s solution were filtered through 0.2 micron filters and delivered with the same extracorporeal circuit described above.
Immediately following fixative perfusion the animals were dissected and 4-5 mm thick coronal sections of organs were cut, placed in glass screw-cap bottles, and transported, as detailed below, for light or electron microscopy.
Straight Frozen Non-ischemic Control
One animal was subjected to straight freezing (i.e., not treated with cryoprotectant). Following induction of anesthesia and intubation the animal was supported on a ventilator while being externally cooled in a crushed ice-water bath. When the EKG documented profound bradycardia at 26°C, the animal was disconnected from the ventilator, placed in a plastic bag, submerged in an isopropanol cooling bath at -10°C, and chilled to dry ice and liquid nitrogen temperature per the same protocol used for the other two experimental groups as described below.
Preparation of FGP Animals
Following placement of cannulae, FGP animals were subjected to total body washout (TBW) by open-circuit perfusion of 500 mL of glycerol-free perfusate. The extracorporeal circuit was then closed and constant-rate addition of glycerol-containing perfusate was begun.
Cryoprotective perfusion continued until the target concentration of glycerol was reached or the supply of glycerol-concentrate perfusate was exhausted.
Preparation of FIGP Animals
In the FIGP animals, ventilator support was discontinued following anesthesia and administration of Metubine. The endotracheal tube was clamped and the ischemic episode was considered to have begun when cardiac arrest was documented by absent EKG.
After the start of the ischemic episode the animals were allowed to remain on the operating table at room temperature ( 22°C to 25°C) for a 30 minute period to simulate the typical interval between pronouncement of legal death in a clinical environment and the start of external cooling at that time. During the 30 minute normothermic ischemic interval the femoral cut-down was performed and monitoring lines were placed in the right femoral artery and vein as per the FGP animals. Prior to placement, the monitoring catheters were irrigated with normal saline, and following placement the catheters were filled with 1000 unit/mL of sodium heparin to guard against clot obstruction of the catheter during the post-arrest ischemic period.
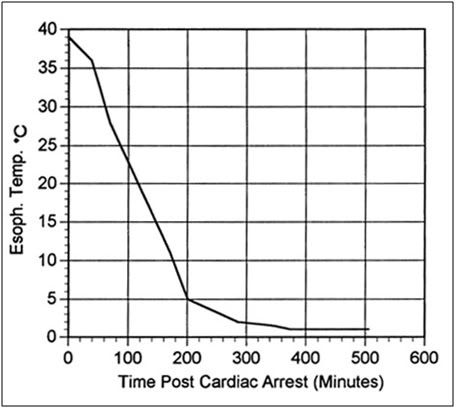
Figure 4: Typical cooling curve of FIGP animals to ~1°C following cardiac arrest.
After the 30 minute normothermic ischemic period the animals were placed in a 1-mil polyethylene bag, transferred to an insulated container in which a bed of crushed ice had been laid down, and covered over with ice. A typical cooling curve for a FIGP animal is presented in Figure 4. FIGP animals were stored on ice in this fashion for a period of 24 hours, after which time they were removed from the container and prepared for perfusion using the surgical and perfusion protocol described above.
Perfusate
TABLE I
Perfusate Composition
Component mM
Potassium Chloride 2.8
Dibasic Potassium Phosphate 5.9
Sodium Bicarbonate 10.0
Sodium Glycerophosphate 27.0
Magnesium Chloride 4.3
Dextrose 11.0
Mannitol 118.0
Hydroxyethyl Starch 50 g/l
The perfusate was an intracellular formulation which employed sodium glycerophosphate as the impermeant species and hydroxyethyl starch (HES)(av. MW 400,000 - 500,000) as the colloid. The composition of the base perfusate is given in Table I. The pH of the perfusate was adjusted to 7.6 with potassium hydroxide. A pH above 7.7, which would have been “appropriate” to the degree of hypothermia experienced during cryoprotective perfusion (9), was not achievable with this mixture owing to problems with complexing of magnesium and calcium with the phosphate buffer, resulting in an insoluble precipitate.
Perfusate components were reagent or USP grade and were dissolved in USP grade water for injection. Perfusate was pre-filtered through a Whatman GFB glass filter (a necessary step to remove precipitate) and then passed through a Pall 0.2 micron filter prior to loading into the extracorporeal circuit.
Perfusion
Perfusion of both groups of animals was begun by carrying out a total body washout (TBW) with the base perfusate in the absence of any cryoprotective agent. In the FGP group washout was achieved within 2 – 3 minutes of the start of open circuit asanguineous perfusion at a flow rate of 160 to 200 mL/min and an average perfusion pressure of 40 mm Hg. TBW in the FGP group was considered complete when the hematocrit was unreadable and the venous effluent was clear. This typically was achieved after perfusion of 500 mL of perfusate.
Complete blood washout in the FIGP group was virtually impossible to achieve (see “Results” below). A decision was made prior to the start of this study (based on previous clinical experience with ischemic human cryopreservation patients) not to allow the arterial pressure to exceed 60 mm Hg for any significant period of time. Consequently, peak flow rates obtained during both total body washout and subsequent glycerol perfusion in the FIGP group were in the range of 50-60 mL/min at a mean arterial pressure of 50 mm Hg.
Due to the presence of massive intravascular clotting in the FIGP animals it was necessary to delay placement of the atrial (venous) cannula (lest the drainage holes become plugged with clots) until the large clots present in the right heart and the superior and inferior vena cava had been expressed through the atriotomy. The chest was kept relatively clear of fluid/clots by active suction during this interval. Removal of large clots and reasonable clearing of the effluent was usually achieved in the FIGP group after 15 minutes of open circuit asanguineous perfusion, following which the circuit was closed and the introduction of glycerol was begun.
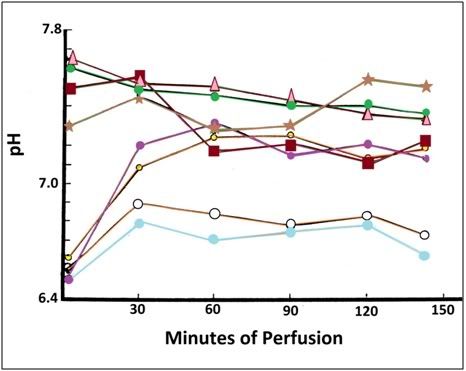
Figure 5: pH of non-ischemic Δ•▪*(FGP) and ischemic ●●● ᴑ (FIGP) cats during cryoprotective perfusion. The FIGP animals were, as expected, profoundly acidotic with the initial arterial pH being between 6.5 and 6.6.
The arterial pO2 of animals in both the FGP and FIGP groups was kept between 600 mm Hg and 760 mm Hg throughout TBW and subsequent glycerol perfusion. Arterial pH in the FGP animals was between 7.1 and 7.7 and was largely a function of the degree of diligence with which addition of buffer was pursued. Arterial pH in the FIGP group was 6.5 to 7.3. Two of the FIGP animals were not subjected to active buffering during perfusion and as a consequence recovery of pH to more normal values from the acidosis of ischemia (starting pH for FIGP animals was typically 6.5 to 6.6) was not as pronounced (Figure 5).
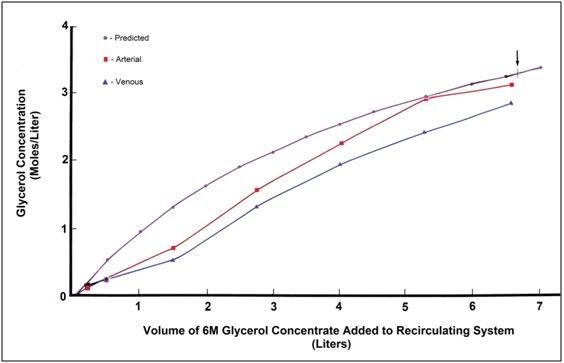
Figure 6: Calculated versus actual increase in arterial and venous glycerol concentration in the FGP animals. Arrow indicates actual time of termination of perfusion.
Introduction of glycerol was by constant rate addition of base perfusate formulation made up with 6M glycerol to a recirculating reservoir containing 3 liters of glycerol-free base perfusate. The target terminal tissue glycerol concentration was 3M and the target time course for introduction was 2 hours. The volume of 6M glycerol concentrate required to reach a terminal concentration in the recirculating system (and thus presumably in the animal) was calculated as follows:
Vp
Mc = ——— Mp
Vc + Vp
where
Mc = Molarity of glycerol in animal and circuit.
Mp = Molarity of glycerol concentrate.
Vc = Volume of circuit and exchangeable volume of animal.*
Vp = Volume of perfusate added.
* Assumes an exchangeable water volume of 60% of the pre-perfusion weight of the animal.
Glycerolization of the FGP animals was carried out at 10°C to 12°C. Initial perfusion of FIGP animals was at 4°C to 5°C with warming (facilitated by TBW with warmer perfusate and removal of surface ice packs) to 10°-12°C for cryoprotectant introduction. The lower TBW temperature of the FIGP animals was a consequence of the animals having been refrigerated on ice for the 24 hours preceding perfusion.
Following termination of the cryoprotective ramp, the animals were removed from bypass, the aortic cannula was left in place to facilitate prompt reperfusion upon rewarming, and the venous cannula was removed and the right atrium closed. The chest wound was loosely closed using surgical staples.
Concurrent with closure of the chest wound, a burr hole craniotomy 3 to 5 mm in diameter was made in the right parietal bone of all animals using a high speed Dremel “hobby” drill. The purpose of the burr hole was to allow for post-perfusion evaluation of cerebralvolume, assess the degree of blood washout in the ischemic animals and facilitate rapid expansion of the burr hole on re-warming to allow for the visual evaluation of post-thaw reperfusion (using dye).
The rectal thermistor probe used to monitor core temperature during perfusion was replaced by a copper/constantan thermocouple at the conclusion of perfusion for monitoring of the core temperature during cooling to -79°C and -196°C.
Cooling to -79°C
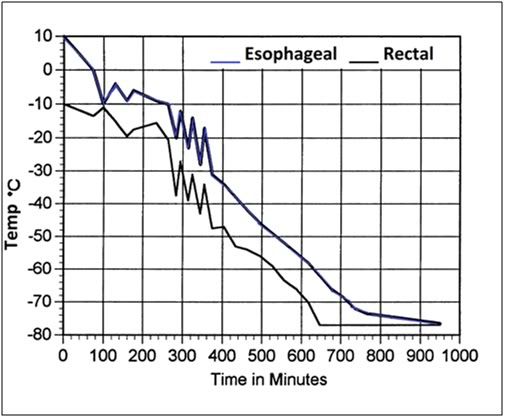
Figure 7: Representative cooling curve (esophageal and rectal temperatures) of FGP and FIGP animals from ~ 10°C to ~ -79°C. The ragged curve with sharp temperature excursions and rebounds is an artifact of the manual control of temperature descent via the addition of chunks of dry ice.
Cooling to -79°C was carried out by placing the animals within two 1 mil polyethylene bags and submerging them in an isopropanol bath which had been pre-cooled to -10°C. Bath temperature was slowly reduced to -79°C by the periodic addition of dry ice. A typical cooling curve obtained in this fashion is shown in Figure 7. Cooling was at a rate of approximately 4°C per hour.
Cooling to and Storage at -196°C
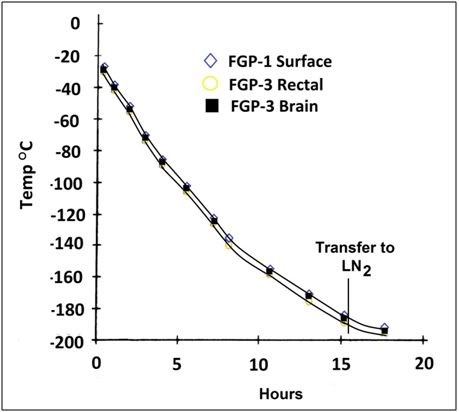
Figure 8: Animals were cooled to -196°C by immersion in liquid nitrogen (LN2) vapor in a Linde LR-40 cryogenic dewar. When a core temperature of ~-180 to -185°C was reached, the animals were immersed in LN2.
Following cooling to -79°C, the plastic bags used to protect the animals from alcohol were removed, the animals were placed inside nylon bags with draw-string closures and were then positioned atop a 6″ high aluminum platform in an MVE TA-60 cryogenic dewar to which 2″-3″ of liquid nitrogen had been added. Over a period of approximately 15 hours the liquid nitrogen level was gradually raised until the animal was submerged. A typical cooling curve to liquid nitrogen temperature for animals in this study is shown in Figure 8. Cooling rates to liquid nitrogen temperature were approximately 0.178°C per hour. After cool-down animals were maintained in liquid nitrogen for a period of 6-8 months until being removed and re-warmed for gross structural, histological, and ultrastructural evaluation.
Re-warming
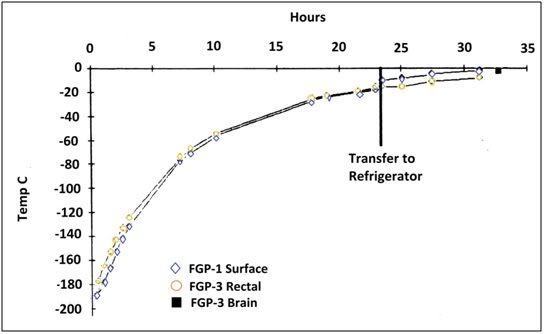
Figure 9: Rewarming of all animals was accomplished by removing the animals from LN2 and placing them in a pre-cooled box insulated with 15.2 cm of polyurethane (isocyothianate) foam to which 1.5 L of LN2 (~2 cm on the bottom of the box) of LN2 had been added. When the core temperature of the animals reached -20°C the animals were transferred to a mechanical refrigerator at 3.4°C.
The animals in both groups were re-warmed to -2°C to -3°C by removing them from liquid nitrogen and placing them in a pre-cooled box insulated on all sides with a 10.2 cm thickness of Styrofoam and containing a small quantity of liquid nitrogen. The animals were then allowed to re-warm to approximately -20°C, at which time they were transferred to a mechanical refrigerator at a temperature of 8°C. When the core temperature of the animals had reached -2°C to -3°C the animals were removed to a bed of crushed ice for dissection, examination and tissue collection for light and electron microscopy. A typical re-warming curve is presented in Figure 9.
Modification of Protocol Due To Tissue Fracturing
After the completion of the first phase of this study (perfusion and cooling to liquid nitrogen temperature) the authors had the opportunity to evaluate the gross and histological condition of the remains of three human cryopreservation patients who were removed from cryogenic storage and converted to neuropreservation (thus allowing for post-arrest dissection of the body, excluding the head) (10). The results of this study confirmed previous, preliminary, data indicative of gross fracturing of organs and tissues in animals cooled to and re-warmed from -196°C. These findings led us to abandon our plans to reperfuse the animals in this study with oxygenated, substrate-containing perfusate (to have been followed by fixative perfusion for histological and ultrastructural evaluation) which was to be have been undertaken in an attempt to assess post-thaw viability by evaluation of post-thaw oxygen consumption, glucose uptake, and tissue-specific enzyme release.
Re-warming and examination of the first animal in the study confirmed the presence of gross fractures in all organ systems. The scope and severity of these fractures resulted in disruption of the circulatory system, thus precluding any attempt at reperfusion as was originally planned.
Preparation of Tissue Samples For Microscopy
Fixation
TABLE II.
Composition Of Modified Karnovsky’s Solution
Component g/l
Paraformaldehyde 40
Glutaraldehyde 20
Sodium Chloride 0.2
Sodium Phosphate 1.42
Calcium Chloride 2.0 mM
pH adjusted to 7.4 with sodium hydroxide.
Samples of four organs were collected for subsequent histological and ultrastructural examination: brain, heart, liver and kidney. Dissection to obtain the tissue samples was begun as soon as the animals were transferred to crushed ice. The brain was the first organ removed for sampling. The burr hole created at the start of perfusion was rapidly extended to a full craniotomy using rongeurs (Figure 14). The brain was then removed en bloc to a shallow pan containing iced, modified Karnovsky’s fixative containing 25% w/v glycerol (see Table II for composition) sufficient to cover it. Slicing of the brain into 5 mm thick sections was carried out with the brain submerged in fixative in this manner. At the conclusion of slicing a 1 mm section of tissue was excised from the visual cortex and fixed in a separate container for electron microscopy. During final sample preparation for electron microscopy care was taken to avoid the cut edges of the tissue block in preparing the Epon embedded sections.
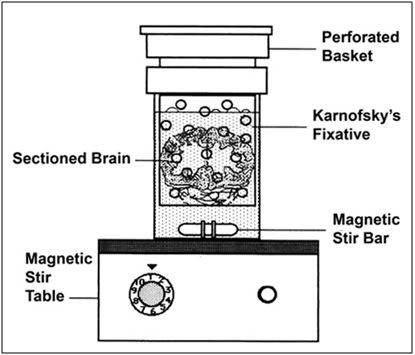
Figure 10: The sagitally sectioned (5 mm thickness) brains of the animals were placed in a perforated basket immersed in Karnofsky’s fixative. This assembly was placed atop a magnetic stirring table and the fixative was gently stirred with a magnetic stirring bar.
The sliced brain was then placed in 350 ml of Karnovsky’s containing 25%w/v glycerol in a special stirring apparatus which is illustrated in Figure 10. This fixation/de-glycerolization apparatus consisted of two plastic containers nested inside of each other atop a magnetic stirrer. The inner container was perforated with numerous 3 mm holes and acted to protect the brain slices from the stir bar which continuously circulated the fixative over the slices. The stirring reduced the likelihood of delayed or poor fixation due to overlap of slices or stable zones of tissue water stratification. (The latter was a very real possibility owing to the high viscosity of the 25%w/v glycerol-containing Karnovsky’s.)
De-glycerolization of Samples
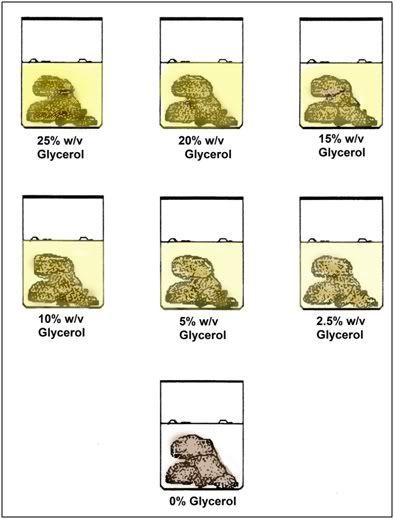
Figure 11: Following fixation, the tissues slices of all organs evaluated by microscopy were serially de-glycerolized using the scheme shown above. When all of the glycerol was unloaded from the tissues they were shipped in modified Karnovsky’s to outside laboratories for histological and electron microscopic imaging.
To avoid osmotic shock all tissue samples were initially immersed in Karnovsky’s containing 25%w/v glycerol at room temperature and were subsequently de-glycerolized prior to staining and embedding by stepwise incubation in Karnovsky’s containing decreasing concentrations of glycerol (see Figure 11 for the de-glycerolization protocol).
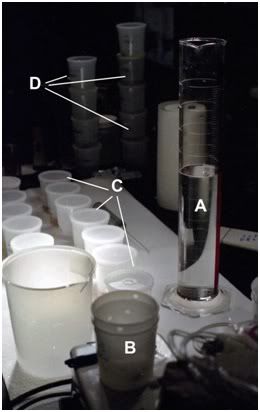
Figure 12: Fixation and de-glycerolization set up employed to prepare tissues for subsequent microscopic examination. Karnofsky’s fixative (A) was added to the tissue slice fixation apparatus (B) and the tissue slices were then subjected to serial immersion in fixative bathing media containing progressively lower concentrations of glycerol (C) (see Figure 11).
To prepare tissue sections from heart, liver, and kidney for microscopy, the organs were first removed en bloc to a beaker containing an amount of ice-cold fixative containing 25% w/v glycerol sufficient to cover the organ. The organ was then removed to a room temperature work surface at where 0.5 mm sections were made with a Stadie-Riggs microtome. The microtome and blade were pre-wetted with fixative, and cut sections were irrigated from the microtome chamber into a beaker containing 200 ml of room-temperature fixative using a plastic squeeze-type laboratory rinse bottle containing fixative solution. Sections were deglycerolized using the same procedure previously detailed for the other slices.
Osmication and Further Processing
At the conclusion of de-glycerolization of the specimens all tissues were separated into two groups; tissues to be evaluated by light microscopy, and those to be examined with transmission electron microscopy. Tissues for light microscopy were shipped in glycerol-free modified Karnovsky’s solution to American Histolabs, Inc. in Rockville, MD for paraffin embedding, sectioning, mounting, and staining.
Tissues for electron microscopy were transported to the facilities of the University of California at San Diego in glycerol-free Karnovsky’s at 1° to 2°C for osmication, Epon embedding, and EM preparation of micrographs by Dr. Paul Farnsworth.
Due to concerns about the osmication and preparation of the material processed for electron microscopy by Farnsworth, tissues from the same animals were also submitted for electron microscopy to Electronucleonics of Silver Spring, Maryland.
III. EFFECTS OF GLYCEROLIZATION
Perfusion of FGP Animals
Blood washout was rapid and complete in the FGP animals and vascular resistance decreased markedly following blood washout. Vascular resistance increased steadily as the glycerol concentration increased, probably as a result of the increasing viscosity of the perfusate.
Within approximately 5 minutes of the beginning of the cryoprotective ramp, bilateral ocular flaccidity was noted in the FGP animals. As the perfusion proceeded, ocular flaccidity progressed until the eyes had lost approximately 30% to 50% of their volume.
Gross examination of the eyes revealed that initial water loss was primarily from the aqueous humor, with more significant losses from the posterior chamber of the eyes apparently not occurring until later in the course of perfusion. Within 15 minutes of the start of glycerolization the corneal surface became dimpled and irregular and the eyes had developed a “caved-in” appearance.
Dehydration was also apparent in the skin and skeletal muscles and was evidenced by a marked decrease in limb girth, profound muscular rigidity, cutaneous wrinkling (Figure 11), and a “waxy-leathery” appearance and texture to both cut skin and skeletal muscle.
Tissue water evaluations conducted on ileum, kidney, liver, lung, and skeletal muscle confirmed and extended the gross observations.
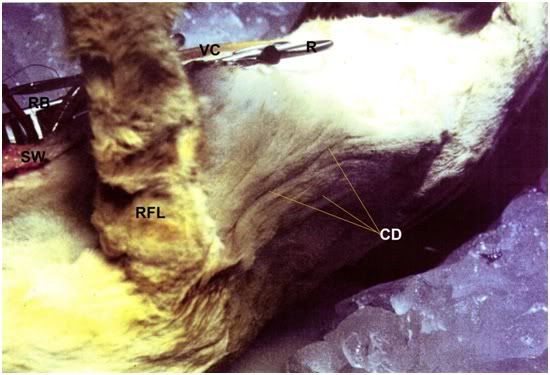
Figure 13: Cutaneous dehydration following glycerol perfusion is evidenced by washboard wrinkling of the thoraco-abdominal skin (CD). The ruffled appearance of the fur on the right foreleg (RF) is also an artifact of cutaneous dehydration. The sternotomy wound, venous cannula and the Weitlaner retractor (R) and the retractor blade (RB) holding open the chest wound are visible at the upper left of the photo.
Preliminary observation suggest that water loss was in the range of 30% to 40% in most tissues. As can be seen in Table III, total body water losses attributable to dehydration, while typically not as profound, were still in the range of 18% to 34%. The gross appearance of the heart suggested a similar degree of dehydration, as evidenced by modest shrinkage and the development of a “pebbly” surface texture and a somewhat translucent or “waxy” appearance.
TABLE III.
Total Water-Loss Associated With Glycerolization of the Cat
____________________________________________________
Animal Pre-Perfusion Post-Perfusion Kg./ % Lost As
# Weight Kg. Weight Water Dehydration
FGP-1 4.1 3.6 2.46 18
FGP-2 3.9 3.1 2.34 34
FGP-3 4.5 3.9 2.70 22
FGP-4 6.0 5.0 3.60 28
FIGP-1 3.4 3.0 2.04 18
FIGP-2 3.4 3.2 2.04 9
FIGP-3 4.32 3.57 2.59 29
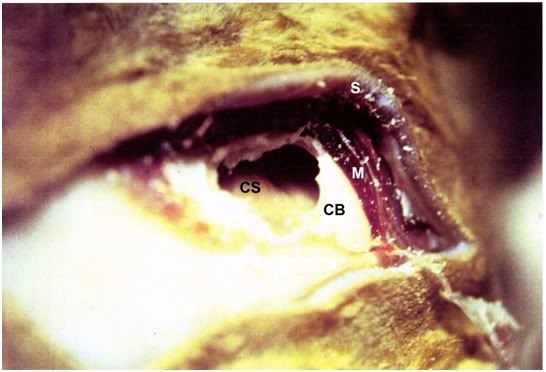
Figure 14: Cerebrocortical dehydration as a result of 4M glycerol perfusion. The cortical surface (CS) is retracted ~5-8 mm below the margin of the cranial bone (CB).
Examination of the cerebral hemispheres through the burr hole (Figure 14) and of the brain in the brain brainpan (Figure 19) revealed an estimated 30% to 50% reduction in cerebral volume, presumably as a result of osmotic dehydration secondary to glycerolization. The cortices also had the “waxy” amber appearance previously observed as characteristic of glycerolized brains.
The gross appearance of the kidneys, spleen, mesenteric and subcutaneous fat, pancreas, and reproductive organs (where present) were unremarkable. The ileum and mesentery appeared somewhat dehydrated, but did not exhibit the waxy appearance that was characteristic of muscle, skin, and brain.
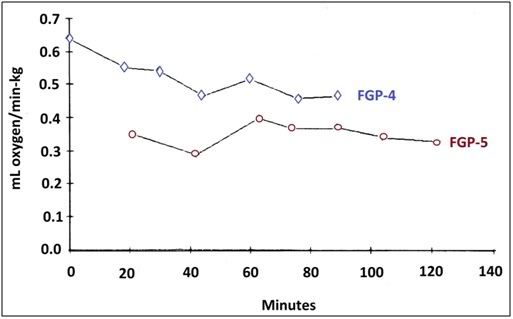
Figure 15: Oxygen consumption was not apparently affected by glycerolization as can be seen in the data above from the perfusions of FGP-5 and FGP-5.
Oxygen consumption (determined by measuring the arterial/venous difference) throughout perfusion was fairly constant and did not appear to be significantly impacted by glycerolization, as can be seen Figure 12.
Perfusion of FIGP Animals
As previously noted, the ischemic animals had far lower flow rates at the same perfusion pressure as FGP animals and demonstrated incomplete blood washout. Intravascular clotting was serious a barrier to adequate perfusion. Post-thaw dissection demonstrated multiple infarcted areas in virtually all organ systems; areas where blood washout and glycerolization were incomplete or absent. In contrast to the even color and texture changes observed in the FGP animals, the skin of the FIGP animals developed multiple, patchy, non-perfused areas which were clearly outlined by surrounding, dehydrated, amber-colored glycerolized areas.
External and internal examination of the brain and spinal cord revealed surprisingly good blood washout of the central nervous system. While grossly visible infarcted areas were noted, these were relatively few and were generally no larger than 2 mm to 3 mm in diameter. With few exceptions, the pial vessels were free of blood and appeared empty of gross emboli. One striking difference which was consistently observed in FIGP animals was a far less profound reduction in brain volume during glycerolization (Figure 17). This may have been due to a number of factors: lower flow rates, higher perfusion pressures, and the increased capillary permeability and perhaps increased cellular permeability to glycerol.
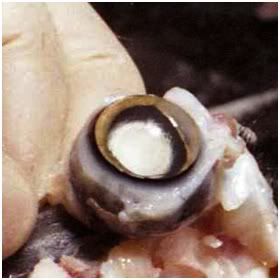
Figure 16: The eye of an FGP animal following cryopreservation. The cornea has become concave due to the glycerol-induced osmotic evacuation of the aqueous humor. The vitreous humor is completely obscured by the lens which has become white and opaque as a result of the precipitation of the crystallin proteins in the lens.
Whereas edema was virtually never a problem during glycerolization of FGP animals, edema was universal in the FIGP animals after as little as 30 minutes of perfusion. In the central nervous system this edema was evidenced by a “rebound” from initial cerebral shrinkage to frank cerebral edema, with the cortices, restrained by the dura, often abutting or slightly projecting into the burr hole. Marked edema of the nictating membranes, the lung, the intestines, and the pancreas was also a uniform finding at the conclusion of cryoprotective perfusion. The development of edema in the central nervous system sometimes closely paralleled the beginning of “rebound” of ocular volume and the development of ocular turgor and frank ocular edema.
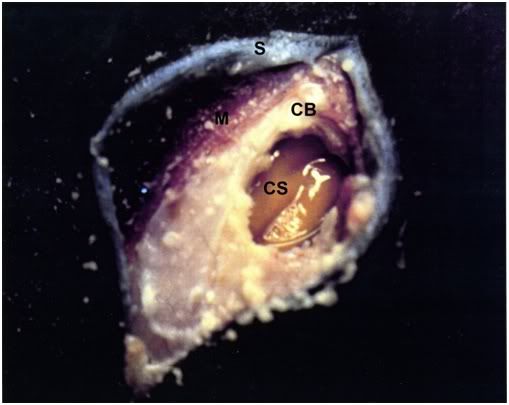
Figure 17: The appearance of the brain of an FIGP animal following cryoprotective perfusion as seen through a craniotomy performed over the right temporal lobe. The cortical surface (CS) is retracted ~3-5 mm from the cranial bone (CB) and appears
In contrast to the relatively good blood washout observed in the brain, the kidneys of FIGP animals had a very dark and mottled appearance. While some areas (an estimated 20% of the cortical surface) appeared to be blood-free, most of the organ remained blood-filled throughout perfusion. Smears of vascular fluid made from renal biopsies which were collected at the conclusion of perfusion (for tissue water determinations) revealed the presence of many free and irregularly clumped groups of crenated and normal-appearing red cells, further evidence of the incompleteness of blood washout. Microscopic examination of recirculating perfusate revealed some free, and a few clumped red cells. However, the concentration was low, and the perfusate microhematocrit was unreadable at the termination of perfusion (i.e., less than 1%).
The liver of FIGP animals appeared uniformly blood-filled throughout perfusion, and did not exhibit even the partial blood washout evidenced by the kidneys. However, despite the absence of any grossly apparent blood washout, tissue water evaluations in one FIGP animal were indicative of osmotic dehydration and thus of some perfusion.
The mesenteric, pancreatic, splanchnic, and other small abdominal vessels were largely free of blood by the conclusion of perfusion. However, blood-filled vessels were not uncommon, and examination during perfusion of mesenteric vessels performed with an ophthalmoscope at 20x magnification revealed stasis in many smaller vessels, and irregularly shaped small clots or agglutinated masses of red cells in most of the mesenteric vessels. Nevertheless, despite the presence of massive intravascular clotting, perfusion was possible, and significant amounts of tissue water appear to have been exchanged for glycerol.
One immediately apparent difference between the FGP and FIGP animals was the accumulation in the lumen of the ileum of large amounts of perfusate or perfusate ultrafiltrate by the ischemic animals. Within approximately 10 minutes of the start of reperfusion, the ileum of the ischemic animals that had been laparotomized was noticed to be accumulating fluid. By the end of perfusion, the stomach and the small and large bowel had become massively distended with perfusate. Figure 14 shows both FIGP and FGP ileum at the conclusion of glycerol perfusion. As can be clearly seen, the FIGP intestine is markedly distended. Gross examination of the gut wall was indicative of tissue-wall edema as well as intraluminal accumulation of fluid. Often by the end of perfusion, the gut had become so edematous and distended with perfusate that it was impossible to completely close the laparotomy incision. Similarly, gross examination of gastric mucosa revealed severe erosion with the mucosa being very friable and frankly hemorrhagic.
Escape of perfusate/stomach contents from the mouth (purging) which occurs during perfusion in ischemically injured human suspension patients did not occur, perhaps due to greater post-arrest competence of the gastroesophageal valve in the cat.
Oxygen consumption in the two ischemic cats in which it was measured was dramatically impacted, being only 30% to 50% of control and deteriorating throughout the course of perfusion (Figure 12).
IV. GROSS EFFECTS OF COOLING TO AND REWARMING FROM -196°C
The most striking change noted upon thawing of the animals was the presence of multiple fractures in all organ systems. As had been previously noted in human cryopreservation patients, fracturing was most pronounced in delicate, high flow organs which are poorly fiber-reinforced. An exception to this was the large arteries such as the aorta, which were heavily fractured.
Fractures were most serious in the brain, spleen, pancreas, and kidney. In these organs fractures would often completely divide or sever the organ into one or more discrete pieces. Tougher, more fiber-reinforced tissues such as myocardium, skeletal muscle, and skin were less affected by fracturing; there were fewer fractures and they were smaller and less frequently penetrated the full thickness of the organ.
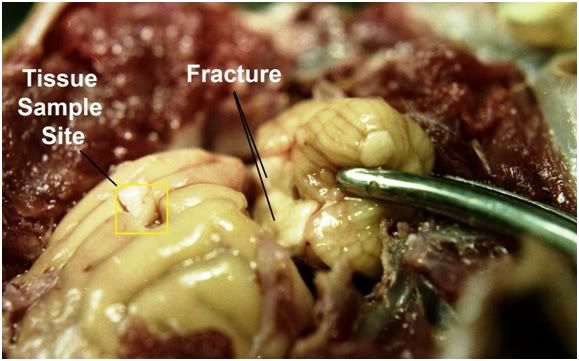
Figure 18: All of the animals in the study exhibited fractures of the white matter that transected the brain between the cerebellum and the cerebral cortices. Similarly, the spinal cord was invariable severed by fractures in several locations and exhibited the appearance of a broken candle stick. The yellow box encloses a sampling area used to determine brain water content.
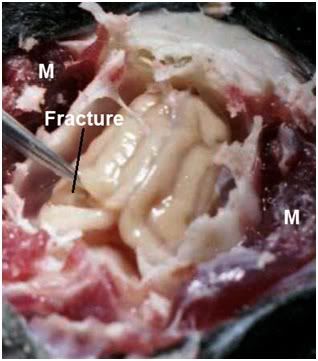
Figure 19: Deep fracture of the left occipital cortex. Note the absence oif fracturing in the adjacent skeletal muscle (M) observed in FGP-1. Note that the brain appears shrunken and retracted in the brainpan.
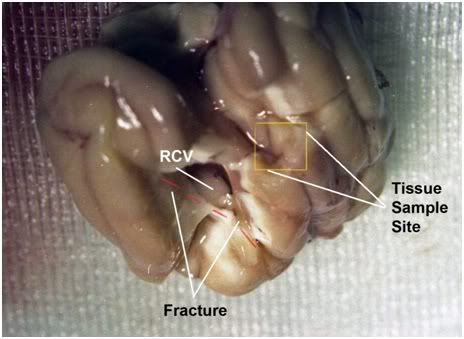
Figure 20: Appearance of the brain after removal from the brainpan. There is a massive fracture of thew right frontal=temporal cortex which penetrates the full thickness of the cerebral hemisphere to expose the right cerebral ventricle observed in FIGP-2. The cortex appears buff colored and gives the appearance of being incompletely washed out of blood.
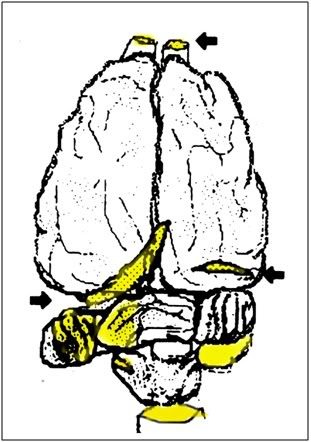
Figure 21: Typical fracture sites in the brain (arrows and yellow shading). The olfactory cortices and the brainstem were invariably completely severed by fractures.
In both FGP and FIGP animals the brain was particularly affected by fracturing (Figures 18, 19 & 22) and it was not uncommon to find fractures in the cerebral hemispheres penetrating through to the ventricles as seen in Figure 20, or to find most of both cerebral hemispheres and the mid-brain completely severed from the cerebellum by a fracture (Figure 18). Similarly, the cerebellum was uniformly severed from the medulla at the foramen magnum as were the olfactory lobes, which were usually retained within the olfactory fossa with severing fractures having occurred at about the level of the transverse ridge. The spinal cord was invariably transversely fractured at intervals of 5 mm to 15 mm over its entire length (Figure 21). Bisecting CNS fractures were most often observed to occur transversely rather than longitudinally. In general, roughly cylindrical structures such as arteries, cerebral hemispheres, spinal cord, lungs, and so on are completely severed only by transverse fractures. Longitudinal fractures tend to be shorter in length and shallower in depth, although there were numerous exceptions to this generalization.
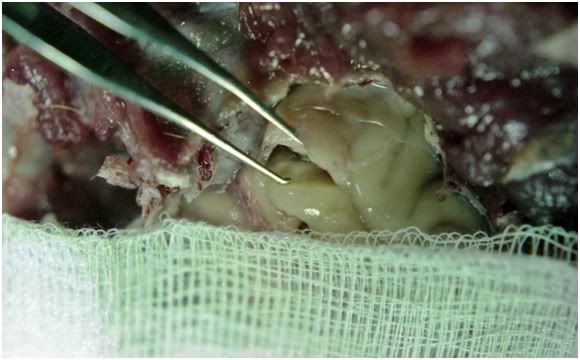
Figure 22: Crisp olfactory lobe fracture which also partially penetrated the pia matter in FGG-4.
In ischemic animals the kidney was usually grossly fractured in one or two locations (Figure 25). By contrast, the well-perfused kidneys of the non-ischemic FGP group exhibited multiple fractures, as can be seen in Figure 24. A similar pattern was observed in other organ systems as well; the non-ischemic animals experienced greater fracturing injury than the ischemic animals, presumably as a result of the higher terminal glycerol concentrations achieved in the non-ischemic group.
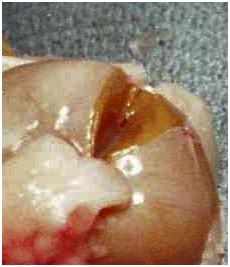
Figure 23: Appearance of a fractured kidney before removal of the renal capsule. The renal capsule has only one fracture, however when the capsule is removed, the extensive fracturing of the renal cortex and medulla become evident (Figure 24, below).
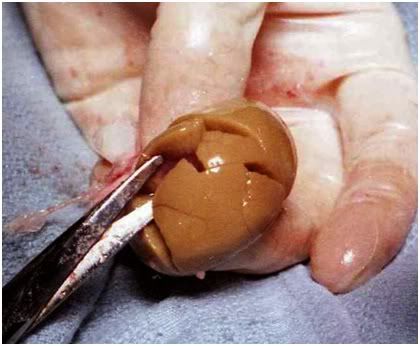
Figure 24: Fractured renal cortex from FGP-1 after removal of the renal capsule. The renal cortex is extensively fractured, the renal medulla slightly less so. Note the uniform, tan/light brown color of the cortex indicating complete blood washout and the absence of red cell trapping.
Cannulae and attached stopcocks where they were externalized on the animals were also frequently fractured. In particular, the polyethylene pressure-monitoring catheters were usually fractured into many small pieces. The extensive fracture damage occurring in cannulae, stopcocks, and catheters was almost certainly a result of handling the animals after cooling to deep subzero temperatures, as this kind of fracturing was not observed in these items upon cooling to liquid nitrogen temperature (even at moderate rates). It is also possible that repeated transfer of the animals after cooling to liquid nitrogen temperature may have contributed to fracturing of tissues, although the occurrence of fractures in organs and bulk quantities of water-cryoprotectant solutions in the absence of handling is well documented in the literature (12, 13).
There were subtle post-thaw alterations in the appearance of the tissues of all three groups of animals. There was little if any fluid present in the vasculature and yet the tissues exhibited oozing and “drip” (similar to that observed in the muscle of frozen-thawed meat and seafood) when cut. This was most pronounced in the straight-frozen animal. The tissues (especially in the ischemic group) also had a somewhat pulpy texture on handling as contrasted with that of unfrozen, glycerolized tissues (i.e., those handled during pre-freezing sampling for water content). This was most in evidence by the accumulation during the course of dissection of small particles of what appeared to be tissue substance with a starchy appearance and an oily texture on gloves and instruments . This phenomenon was never observed when handling fresh tissue or glycerolized tissue prior to freezing and thawing.
There were marked differences in the color of the tissues between the three groups of animals as well. This was most pronounced in the straight-frozen control where the color of almost every organ and tissue examined had undergone change. Typically the color of tissues in the straight-frozen animal was darker, and white or translucent tissues such as the brain or mesentery were discolored with hemoglobin released from lysed red cells.
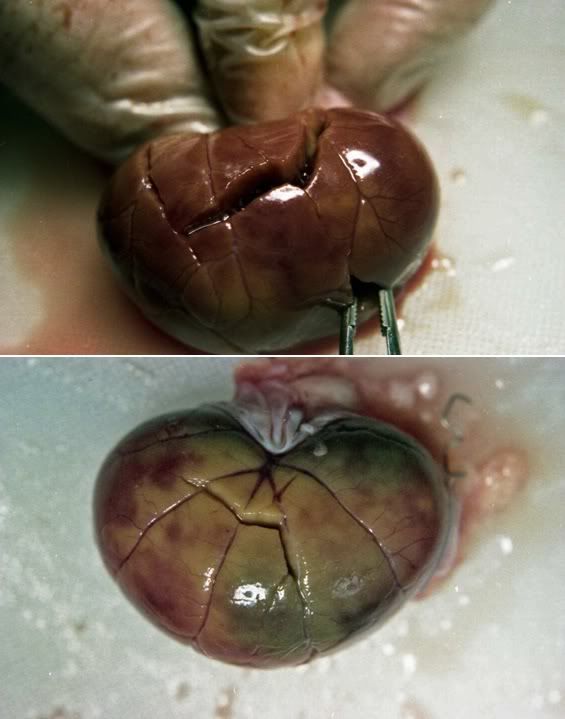
Figure 25: The (ventral) dependent and dorsal (less dependent) surfaces of the right kidney from FIGP-1. There is extensive mottling evidencing incomplete blood washout despite perfusion with many liters of CPA solution. Fracturing is much less extensive than that observed in FGP animals not subjected to prolonged periods of post-arrest ischemia. Note the pink colored “drip” from the organ that is present on sectioning board.
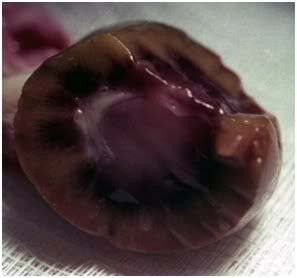
Figure 26: Appearance of the kidney from FIGP-1 shown above on cross-section. The renal medulla appears congested and blood filled.
The FGP and FIGP groups did not experience the profound post-thaw changes in tissue color experienced by the straight-frozen controls, although the livers and kidneys of the FIGP animals appeared very dark, even when contrasted with their pre-perfusion color as observed in those animals laparotomized for tissue water evaluation.
END OF PART 1

Mike, Where did you get these cats?
Gabi
If I recall correctly, 3 sources: pound seizure (http://www.idausa.org/facts/poundseizure.html), class B USDA dealers and in a couple of cases, relinquishment of companion animals to us for research (pets) that were scheduled for euthanasia . This was 30 years ago and the culture and the law were in very different places than they are now. Whilst pound seizure is still technically legal in many locales, virtually no research animals come from this source today in the US. Instead, such animals are simply killed and often rendered into soap, as this allows the “shelters” to recover some of their costs. And that’s a lot of soap since ~3-4 million dogs and cats are destroyed in the US each year (http://www.humanesociety.org/issues/pet_overpopulation/facts/overpopulation_estimates.html). No one in the doggy and kitty Dachau industry will fess up to the end use of the carcasses – however, many of the intermediaries in the disposal chain ultimately sell the carcasses, rendered or not, to China . Stray and feral cats, in particular, constitute a source of tremendous ecological damage; in Australia they have wrecked the avian and small reptile ecosystems. In the US, it is estimated that ~ 1 billion birds are killed each by domestic cats – a disproportionate number of which cats are abandoned, allowed to roam free, or are feral. — Mike Darwin