IV. EFFECTS OF CRYOPRESERVATION ON THE HISTOLOGY OF SELECTED TISSUES (Liver and Kidneys)
Histology was evaluated in two animals each from the FIG and FIGP groups, and in one control animal. Only brain histology was evaluated in the straight-frozen control animal.
Liver
The histological appearance of the liver in all three groups of animals was one of profound injury. Even in the FGP group, the cellular integrity of the liver appeared grossly disrupted. In liver tissue prepared using Yajima stain, the sinusoids and spaces of Disse were filled with flocculent debris, and it was often difficult or impossible to discern cell membranes (Figures 30-32). The collagenous supporting structures of the bile canaliculi were in evidence and the nuclei of the hepatocytes appeared to have survived with few alterations evident at the light level, although frequent pyknotic nuclei were noted in the FIGP group (Figures 31 & 34). Indeed, the nuclei often appeared to be floating in a sea of amorphous material (Figure 34). Not surprisingly, the density of staining of the cytoplasmic material was noticeably reduced over that of the fixative-perfused control. Few intact capillaries were noted.
FGP liver tissue prepared with PAS stain exhibited a similar degree of disruption (Figure 32). However, quite remarkably, the borders of the hepatocytes were defined by a clear margin between glycogen granule containing cytoplasm and non-glycogen containing membrane or other material (membrane debris?) which failed to stain with Yajima due to gross physical disruption, or altered tissue chemistry (Figure 35).
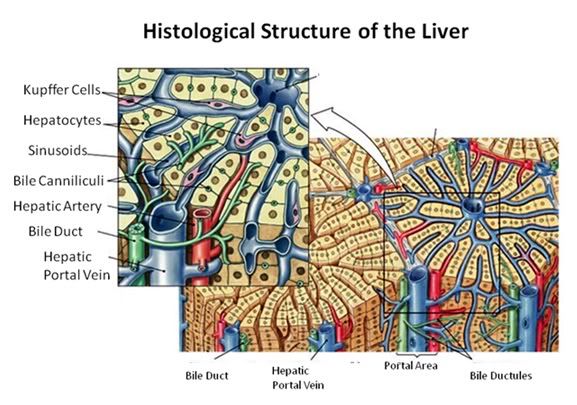
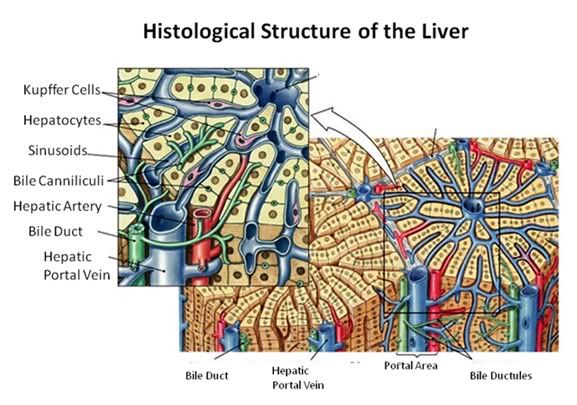
Figure 27: The fundamental histological structural unit of the liver is the liver lobule, a six-sided prism of tissue ~ 2 mm long and ~1 mm in diameter. The lobule is defined by interlobular connective tissue which is not very visible under light microscopy in the cat (or in man). In the corners of the lobular prisms are the portal triads. In tissue cross sections prepared for microscopy, the lobule is filled by cords of hepatic parenchymal cells, the hepatocytes, which radiate from the central vein and are separated by vascular sinusoids. The bulk of the liver consists of epithelial hepatocytes arranged into cords, separated by the vascular sinusoids through which the portal blood percolates. The epithelium of the sinusoids is decorated with phagocytic Kuppfer cells that are the primary mechanism for removing gut bacteria present in the venous splanchnic circulation.
The cords of hepatocytes comprise the hepatic parenchyma. In section, the hepatic cords appear as linear ropes (or cords) of hepatocytes. Viewed 3-dimensionaly, the cords consist of intricately folded branching and connected planes of cells which extend parallel to the long axis of the lobule and radiate out from the its center. The hepatocytes in each cord are attached to each other wherever they come into contact, as well as to the sinusoids at either end of the lobular pyramid. The sinusoids are vascular spaces lined by fenestrated endothelium that has no basement membrane, thus allowing the plasma to pass over the large surface area sheets of hepatocytes for detoxification. The sinusoid endothelium stands off from the underlying hepatocytes allowing space for the plasma to interact with the hepatocytes and Kupffer cells (the space of Disse).
Bile canaliculi, formed by apical surfaces of adjacent hepatocytes, form a network of tiny passages contained within each hepatic cord.
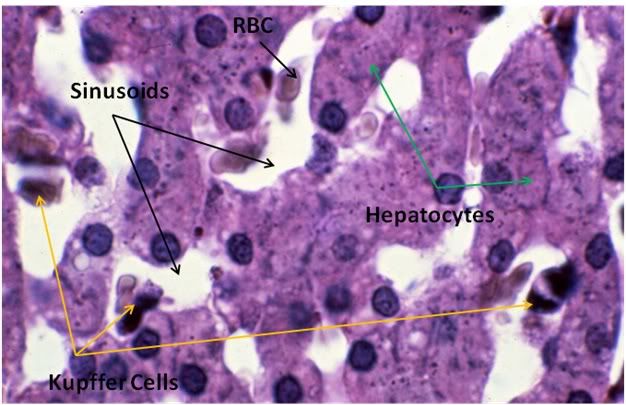 Figure 28: Control-1 Liver, Yajima, 100x. Liver sections from the Control animal demonstrated normal morphology as can be seen in the image above.
Figure 28: Control-1 Liver, Yajima, 100x. Liver sections from the Control animal demonstrated normal morphology as can be seen in the image above.
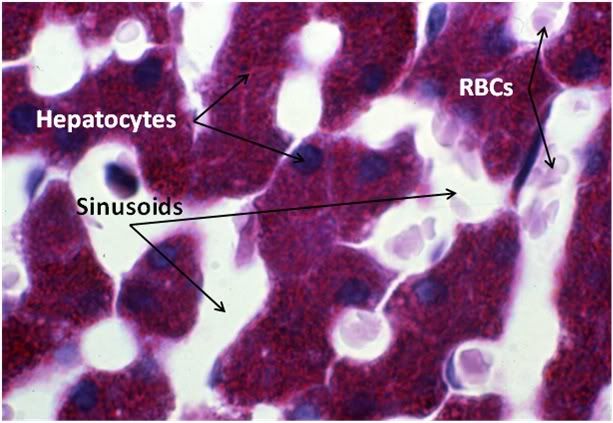
Figure 29: Control-1 Liver, PAS, 100x. Liver sections were prepared with both Yajima and PAS stain in order to allow visualization of structures that neither stain discloses alone; in this case, most importantly, the presence or glycogen granules in the hepatocytes of the Control animal. Note the presence of normal intralobular architecture with crisp cell membranes in evidence, normal appearing sinusoid spaces, and residual sinusoidal red blood cells (RBCs) not washed out during fixative perfusion.
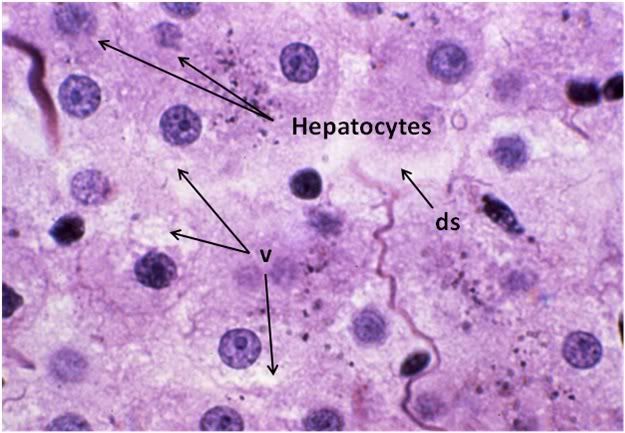 Figure 30: FGP-1 Liver, Yajima, 100x. The livers of FGP animals demonstrated extensive histological disruption. The sinusoids were all but obliterated and appeared filled with debris (ds) and the cytoplasm was extensively vacuolated (v).
Figure 30: FGP-1 Liver, Yajima, 100x. The livers of FGP animals demonstrated extensive histological disruption. The sinusoids were all but obliterated and appeared filled with debris (ds) and the cytoplasm was extensively vacuolated (v).
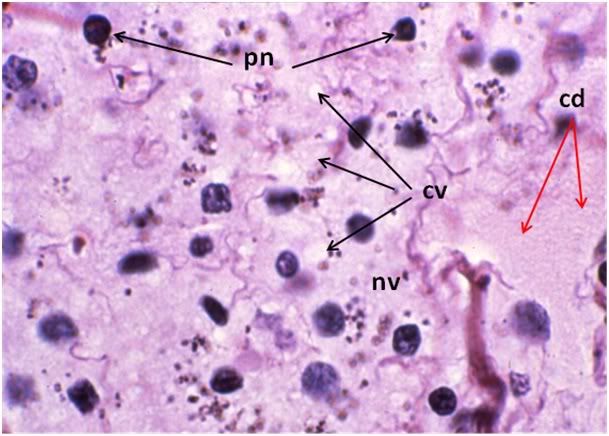 Figure 31: FIG-2 Liver, Yajima, 100x. As was the case with the FGP animals, the sinusoids were barely discernable and appeared filled with cellular debris (cd). In addition to extensive cytoplasmic (cv) and nuclear vacuolization (nv), pyknotic nuclei (pn) were also present. Cell membranes were difficult to discern and in many areas, frank cell lysis appears to have occurred with flocculent cellular debris (cd) appearing to fill the sinusoids.
Figure 31: FIG-2 Liver, Yajima, 100x. As was the case with the FGP animals, the sinusoids were barely discernable and appeared filled with cellular debris (cd). In addition to extensive cytoplasmic (cv) and nuclear vacuolization (nv), pyknotic nuclei (pn) were also present. Cell membranes were difficult to discern and in many areas, frank cell lysis appears to have occurred with flocculent cellular debris (cd) appearing to fill the sinusoids.
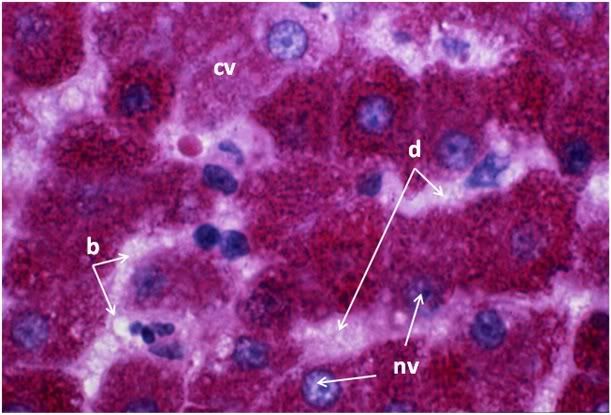 Figure 32: FGP-1, Liver, PAS, 100x. The intensely red-stained granules present in the cytoplasm of the hepatocytes are glycogen deposits selectively stained by PAS. There is extensive cytoplasmic (cv) and nuclear vacuolization (nv) and the sinusoids appear filled with flocculent cellular debris (d). Indeed, it is only possible to discern the outlines of the original individual hepatocytes from the pattern of the intracellular glycogen granules disclosed by the PAS stain.
Figure 32: FGP-1, Liver, PAS, 100x. The intensely red-stained granules present in the cytoplasm of the hepatocytes are glycogen deposits selectively stained by PAS. There is extensive cytoplasmic (cv) and nuclear vacuolization (nv) and the sinusoids appear filled with flocculent cellular debris (d). Indeed, it is only possible to discern the outlines of the original individual hepatocytes from the pattern of the intracellular glycogen granules disclosed by the PAS stain.
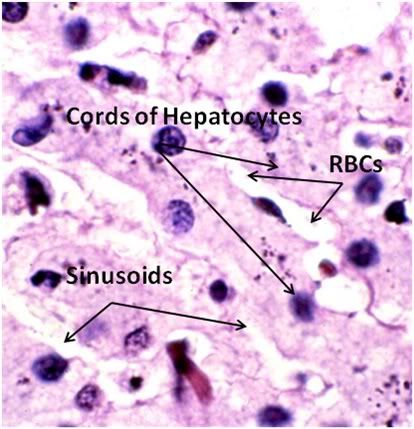
Figure 33: FIG-2, Liver, Yajima, 100x, well preserved area. While the bulk of the hepatic parenchyma exhibited the severe injury seen in Figures 30-32, there were frequently observed islands of comparatively well preserved tissue visible in both the FGP and FIGP sections suggesting that freezing injury is occurring non-homogenously.
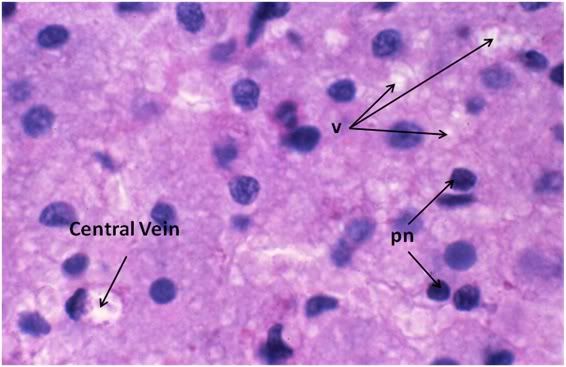
Figure 34: FIG-2, Liver, PAS, 100x, necrotic area. There were patchy areas of frank necrosis visible in the livers of the FIGP animals that were not present in the livers of the FGP animals. This area, adjacent to a central vein, shows extensive cell lysis with heavy vacuolization of the cytoplasm (v) and many pyknotic nuclei (pn) in evidence.
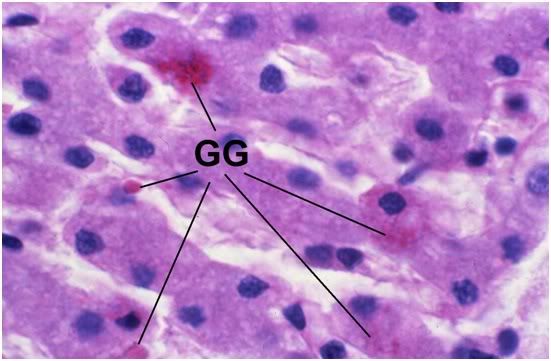
Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Interestingly, in this comparatively well preserved area of FIGP liver it is possible to see some remaining deposits of glycogen that were not consumed during the long post-arrest ischemic interval. The absence of pyknotic nuclei and the relative absence of large intracellular vacuoles is also remarkable.
Kidney
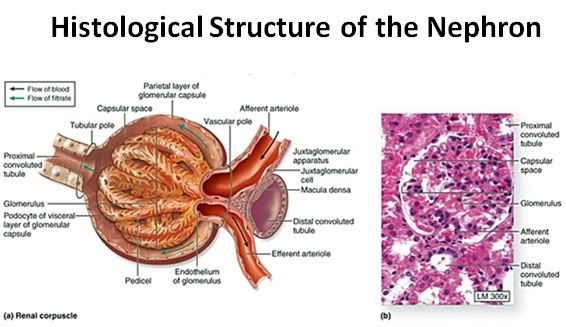
Figure 36: The functional unit of the kidney is the nephron, consisting of the glomerulus and the uriniferous tubule ( the renal corpuscle: a).The capillary tuft of the nephron, the glomerulus, is enclosed within a double cell layered structure; Bowman’s capsule. Bowman’s capsule and the capillary tuft it encloses comprise the glomerulus. Bowman’s capsule and the glomerular capillary tuft constitute the renal (or Malpighian) corpuscle (b).
Bowman’s capsule opens into the proximal convoluted tubule which leads to the loop of Henle. The loop of Henle leads to the distal convoluted tubule which then leads to the collecting duct.
The inner layer of Bowman’s capsule is the visceral layer. It consists of cells called podocytes. The outer layer of Bowman’s capsule is the parietal layer. The pedicels are the foot processes on the podocytes.
The juxtaglomerular cells secrete renin which is ultimately metabolized into angiotensin II, a potent vasoconstrictor critical to maintaining normotension. The macula densa are specialized cells in the distal convoluted tubule that are responsible for sodium, and thus fluid regulation. The juxtaglomerular cells and macula densa make up the juxtaglomerular apparatus.
PAS stain was used to prepare the control, FGP and FIGP renal tissue for light microscopy. The histological appearance of FGP renal tissue was surprisingly good (Figures 329, 40 & 41). The glomeruli and tubules appeared grossly intact and stain uptake was normal. However, a number of alterations from the appearance of the control were apparent. The capillary tuft of the glomeruli appeared swollen and the normal space between the capillary tuft and Bowman’s capsule was absent. There was also marked interstitial edema, and marked cellular edema as evidenced by the obliteration of the tubule lumen by cellular edema.
By contrast, the renal cortex of the FIGP animals, when compared to either the control or the FGP group, showed a profound loss of detail, absent intercellular space, and altered staining (Figures 40 & 42). The tissue appeared frankly necrotic, with numerous pyknotic nuclei and numerous large vacuoles which peppered the cells. One striking difference between FGP and FIGP renal cortex was that the capillaries, which were largely obliterated in the FGP animals, were consistently spared in the FIGP animals. Indeed, the only extracellular space in evidence in this preparation was the narrowed lumen of the capillaries, grossly reduced in size apparently as a consequence of cellular edema.
Both ischemic and non-ischemic sections showed occasional evidence of fracturing, with fractures crossing and severing tubule cells and glomeruli (Figure 41).
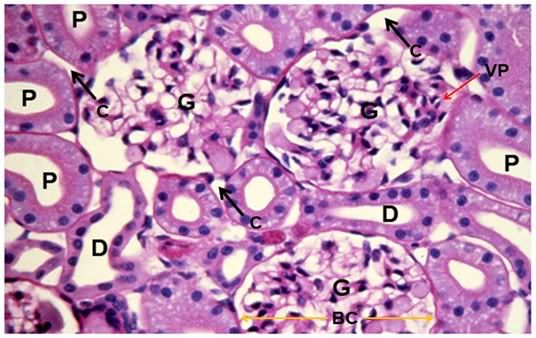
Figure 37: Control, Renal Cortex, PAS 40x. Three glumeruli are present (G) adjacent to crisp, well defined proximal (P) and distal (D) convoluted tubules. The intertubular capillaries (C) show normal diameter with lumens free of red cells or debris. There is normal capsular space between Bowman’s capsule (BC, yellow arrows) and the glomerular capillary tuft and vascular pole (VP) are also normal in appearance.
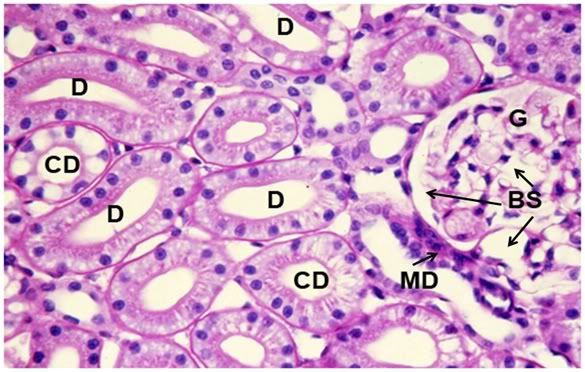
Figure 38: Control-1, PAS 40x. Collecting ducts (CD), distal tubules (D) and a glomerulus (G) are present in 6this micrograph of renal apical column. At right, a glomerulus is present with normal Bowman’s space (BS) and the macula densa (MD) in evidence.
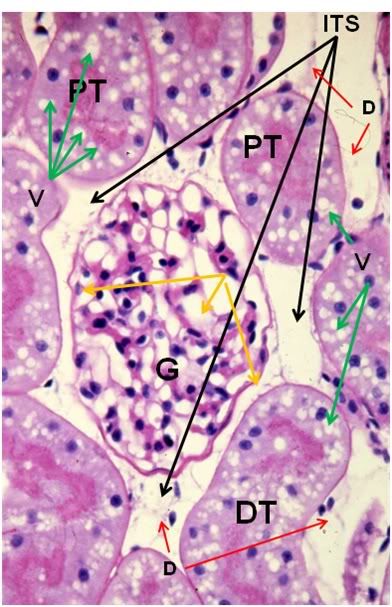
Figure 39: FGP-1, Renal Cortex, PAS, 40x. The intertubular space (ITS) is great expanded and the tubule cells are heavily vacoulated (V) and lack definition. The intratubular space (IS) is no longer in evidence and the architecture of the glomerluar capillary tuft (GT) is radically altered and there is an absence of the normal architecture of Bowman’s space (yellow arrows). The intertubular capillaries appear to have been reduced to debris (D) visible in the intertubular spaces.
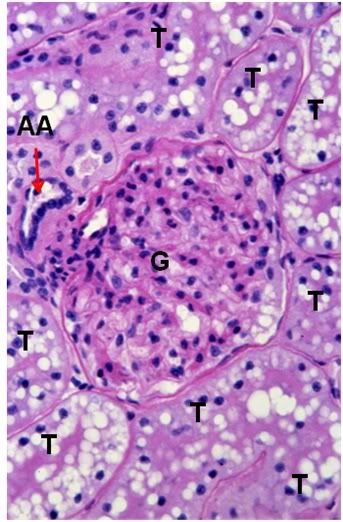
Figure 40: FIG-2 Renal Cortex, PAS, 40x. There is massive cellular edema present with almost complete obliteration of Bowman’s space. The tubule (T) lumens are no longer visible and the tubule cells are extensively vacoulated with many pyknotic nuceli in evidence. Individual tubular cell membranes are impossible to resolve. The afferent glomerular arteriole (AA) appears intact (red arrow).
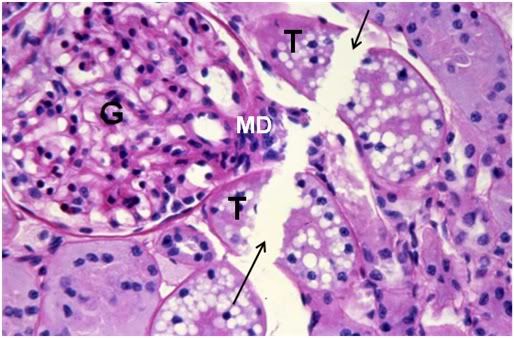
Figure 41: FIG-2 Renal Cortex, fracture present (arrows), PAS, 40x. Two renal tubules, possibly a proximal and distal convoluted tubule (T) are dissected by a fracture as is the macula densa (MD) of the glomerulus (G). Remarkably, there is still a small amount of intertubular space present in this micrograph.
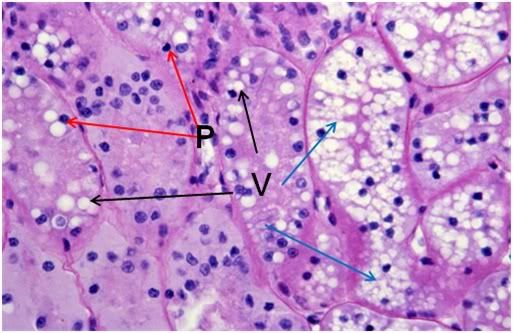
Figure 42: FIG-2 Renal Cortex, PAS 40x. vacuolization (black arrows) and extensive vacuolization (blue arrows) accompanied by necrotic changes, such as the frequent presence of pyknotic nuclei (red arrows).
END OF PART 2

In light of such wide-spread damage to the body during cryopreservation, it seems to me likely that future successful revival techniques will focus mainly on the brain, preferring to regrow the body instead of trying to repair cryogenic damage.
Are the studies on the cats on-going or was this completed a while ago?
Do you have any thoughts about http://www.brainpreservation.org and their different approach? Clearly with their approach, a full-blown mind uploading technology would be required for successful revival as opposed to molecular repair bots.
In light of such wide-spread damage to the body during cryopreservation, it seems to me likely that future successful revival techniques will focus mainly on the brain, preferring to regrow the body instead of trying to repair cryogenic damage.
The same conclusion was reached by a handful of cryonicists circa 1973-4. I was one of them. We thus decided that it was both wasteful and dangerous to haul our cryoinjured, aging and disease ravaged bodies across the decades or centuries for repair and rescue. Our solution was “head only” cryonics (neuropreservation).
Are the studies on the cats on-going or was this completed a while ago?
If you read the paper carefully, you will see that the work was done ~30 years ago. This work was a part of a series of planned studies to delineate the nature and extent of cryoinjury in human cryopatients. The first phase of the study was done using rabbits and was undertaken in Indianapolis, IN in the late 1970s. The Dora Kent crisis in the mid-1980s (http://www.alcor.org/Library/html/DoraKentCase.html) derailed further work. Dora Kent was followed by the cryopreservation of one of the Principal Investigators in 1991, and my departure from Alcor. In the early to mid-1990s, my colleagues and I undertook a study using dogs (http://yarchive.net/med/canine_cryopreservation.html) to simulate the application of cryopreservation techniques under optimum condition (e.g., very little ischemia) using 7.5 M glycerol. This study yielded vastly superior preservation and you can see the micrographs and the paper (minus supporting materials and methods data) at this link: http://www.alcor.org/Library/html/braincryopreservation1.html
Do you have any thoughts about http://www.brainpreservation.org and their different approach? Clearly with their approach, a full-blown mind uploading technology would be required for successful revival as opposed to molecular repair bots.
To quote Yogi Berra, I think it’s de ja vu all over again. IMO, it is a sign, more than anything else, of the failure of contemporary cryonics to properly dominate this intellectual realm and thus to be inclusive of other good minds who’ve also reached the conclusion that biological structure = identity = redefinition of death = possible indefinite survival. Fixation has been carefully considered by cryonicists, almost since the beginning. It faces three monumental problems not confronted by cryonics:
1) Fixatives are comparatively large molecules which must DIFFUSE into tissues before they can act. Under ideal conditions (no ischemia) this not a problem, because no cell is located more than 100 microns from a capillary (http://www.tcd.ie/bioengineering/documents/MBEC_Sara_Checa.pdf). Once ischemia has occurred, even a period of as little as 10-15 minutes, this distance will be increased by many orders if magnitude in the brain (and in other body organs). Diffusion of fixatives under such conditions is typically not fast enough to prevent autolysis. (See: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC495013/pdf/jclinpath00426-0080.pdf, http://publish.uwo.ca/~jkiernan/formglut.htm and: Medawar PB. The rate of penetration of fixatives. J R Microsc Soc. 1941; 61,46, Helander, KG. Kinetic studies of formaldehyde binding in tissue. Biotechnique and Histochemistry. 1994; 69, 177 -179; Helander, K.G. Formaldehyde binding in Brain and Kidney: A kinetic study of fixation; Fox CH., et.al. Formaldehyde fixation. J Histochem. Cytochem. 1985; 33, 845 -853.).
By contrast, thermal diffusion (cooling) is much more rapid. Under ideal conditions, fixation should yield very good structural preservation.However, obtaining those ideal conditions will mean the use of the same costly, complex and logistically daunting procedures to minimize/eliminate ischemic injury now being employed by Alcor.
2) Fixation alone does not yield stable preservation. For that, it is necessary to SOLIDIFY the system. This is typically done by substituting all of the water in the tissues with a chemical or chemicals that can then be plasticized. Typically, monomers, or short chain polymers are used, such as an epoxy (like Epon) resin and then a plasticizer is added to catalyze the polymerization reaction, solidifying the system. Diffusion times for such molecules are very, very long and before they can be introduced, the specimen must be completely dehydrated by prolonged soaking in a solvent, such as acetone or methanol. The Journal of Histotechnology. 1999; 22(4), 317-318. Alternatively, a water driven reaction, such as that used in silicone plastination of human remains for anatomy displays ( a la Gunterr Hagens) may be used. Such a procedure takes weeks or months for an object the size of a human brain and would be very costly.
More to the point, it has yet to be demonstrated that such a procedure is actually workable for an object the size of a human brain; in other words, there is currently no procedure or protocol available that has been demonstrated to work! That is a formidable obstacle, indeed, and that is why there is an “x-prize” type of wager for its development currently on offer.
Most plasticizing reactions, especially those that yield solid, highly stable plastic end products, are exothermic and this means that a large specimen, with an unfavorable surface to volume ratio for heat dissipation (such as an entire organ, like the human brain), may actually reach the ignition point in the interior! Additionally, most plastics are not stable over time and have very active chemistries at or near ambient temperature. A vast sub-discipline exists within polymer chemistry that aims to find ways to increase the stability and durability of plastics and there are many, many journals that chronicle these efforts: http://www.chemseer.com/journals/journals_polymer.shtml. I have been a subscriber to some of these for many years (for other reasons) and I am impressed with the dynamicity of the chemistry of most plastics at ambient temperature.
3) You may be more than you “connectome.” In other words, the biochemistry of the neurons and their synapses may be crucial to personal identity. Fixation radically alters the biochemistry of living systems and does so in complex ways we are now only beginning to understand. Many of these changes involve large alterations in the stereospecificity of biomolecules and in their relationship to each other – changes that are not, in principle, necessarily either reversible or inferrable. — Mike Darwin
Thank you for your analysis. I hadn’t understood that fixation still isn’t adequate for human brains. It’s scary to contemplate the interior of a brain going up in flames!
I’m studying the papers you referred to, and am thinking a lot about planning cryogenics arrangements and my personal hurdles.
The Dora Kent case is a disturbing read, it reminds me that unfortunately that cryogenics still have a long way to go before more widespread public acceptance.
Er did I really just typed cryogenics instead of cryonics?
Sorry, my fingers were faster than my cerebral cortex.
I’m not sure that fixation isn’t a viable approach. It is certainly true that the technology to implement it has not been developed to a marketable state. This may be because there really are very difficult technical and theoretical problems to be overcome, or it may be simply because no one has done the bench work. You can certainly lock up structure homogeneously throughout the brain by perfusing the right fixatives. You need to fix the lipids as well the proteins – unless you can solidify reasonably promptly. However, the REAL issue for stable ambient temperature biopreserevation is to find a way to solidify the system in a timely fashion. This can certainly be done with things like paraffin wax and epoxies – and it can even be done to whole brains and indeed to whole humans, as was demonstrated by the Spanish pathologist Pedro Ara in the 1940s.
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Arasheadofapeasant1.png
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Arasheadofapeasant2.png
The photos above are of the head of Spanish peasant that Ara prepared in the first half of the last century. They have resided for decades in the hot, stuffy Museo de Anatomí Pedro Ara in Cordoba, Argentina. Also in the museum are others of Ara’s preparations, including this striking one of a human head with exposed brain:
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Arabrain.png
Ara is most famous for his postmortem preservation of Eva Peron, the charismatic wife of the Argentine dictator Juan Peron.
http://i293.photobucket.com/albums/mm55/mikedarwin1967/PedroArawithAvaPeron.jpg
http://i293.photobucket.com/albums/mm55/mikedarwin1967/PedroArawithAvaPeron.jpg
http://i293.photobucket.com/albums/mm55/mikedarwin1967/EvaPeronAPSkullFilm002.jpg
There has been a great deal of press and commentary that Ara did not, in fact, preserve Eva Peron, but rather, substituted a wax dummy. Or that he gutted her, as was done to Lenin. This is not the case and the evidence that Peron was preserved intact has special relevance to the issue of ambient temperature preservation, because it shows that the the brain, within the head, can be indefinitely preserved, at least in bulk. The evidence is this anterior posterior skull film made of Eva’s head many years after her preservation:
http://i293.photobucket.com/albums/mm55/mikedarwin1967/EvaPeronSkullNormalSkullFilmcopy.jpg
When I saw this for the first time in the early 1980s, my reaction was, “Aha! He really did do it!” The reason for this is because very, very few people (outside of cryonics) would know what the AP X-ray of a human head/brain would like that had been perfused with a ~15% v/v glycerol solution. The use of a glycerol-based initial flush of the vasculature is unique to Ara’s method (as far as I know) and it is ideally suited to overcome the no-reflow phenomenon in the brain and achieve adequate blood washout and recruitment of closed capillaries in ischemic subjects. In Eva Peron’s case, there was very little ischemia since Ara began perfusion within minutes of her death. Again, these outstanding results are consistent with little or postmortem delay (ischemia).
The truly impressive thing is that he was apparently able to achieve paraffin-impregnation of the brain within the skull. Or, he was otherwise able to achieve stable, long term preservation. It is possible to see more detail in Eva Peron’s brain decades after her death on a plain film X-ray than it is in a 24-hour old corpse with MRI (as an example, gray/white matter differences disappear within hours of death)!
By contrast, Lenin, who was not solidified, and was eviscerated, looks in dreadful condition during one of his recent (and regular) “tune-ups.”:
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Lenin02.png
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Lenin07.png
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Lenin04.png
Yes, Lenin looks impressively preserved when on display:
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Lenin09.png
However, the biological reality is quite different. As an aside and in fairness, I should note that Lenin’s brain, or at least much of it, is indeed in the solid state, since it was sectioned and prepared as standard light microscopy slides using H&E staining. When I visited Lenin’s tomb several years ago, I was surprised at the pungent and all-permeating odor of of formalin and phenol which the savvy visitor can smell as soon as he enters the structure. And it is one incredibly impressive structure – I would rank it with the most impressive of those religious structures I’ve visited: The Vatican is impressive in one way, Lenin’s tomb in quite another…
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Lenin10.png
http://i293.photobucket.com/albums/mm55/mikedarwin1967/Lenin08.png
So, the real work to validate ambient temperature preservation techniques, with or without “chemical fixation,” will be by recourse to the bench – by doing lots of careful experiments. The result will likely be something that could properly be described as “art” as much as science.
And this might well be worth pursuing, if for no other reason than that such preparations should be stable indefinitely – indeed done well, for tens of millions of years – and without refrigeration. Both Eva Peron and Comrade Lenin have long outlasted the then seemingly more durable regimes, institutions, and people who preserved them. That’s quite a lesson, and one we cryonicists would do well to pay attention to. — Mike Darwin