By Mike Darwin

Figure 1: All you need is a pair of hands?
Introduction
There are, at a minimum, five obstacles that must be overcome in order to allow the restoration of human cryopreservation patients to life and health:
- Reversal of any ischemia or ischemia-reperfusion injury (IRI) suffered during the peri- and post-cardiac arrest intervals before definitive stabilization in the solid state can be undertaken.
- Repair of injuries, gross, microscopic, ultramicroscopic and biochemical, secondary to the cryopreservation process.
- Cure for the underlying pathology(ies) which caused the patient’s terminal illness.
- Replacement of any missing or discarded tissues/organs resulting from medical interventions (i.e., amputation, excision, ablation) or from neuropreservation.
- Control over and reversal of the aging process.
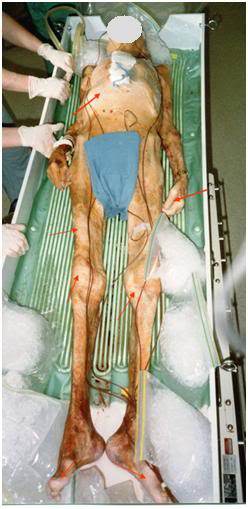 Figure 2: The red arrows in the photo above point to areas of completely failed cryoprotective perfusion in the patient’s skin and peripheral tissues. The cause of these infarcts is post-arrest red blood cell (RBC) aggregation and micro- and macrovascular clotting secondary to prolonged warm and cold ischemia. While this patient received prompt post-arrest manual cardiopulmonary support (CPS), anticoagulation (50,000 IU sodium heparin) and external cooling (ice packs) these efforts were insufficient to prevent global vascular obstruction sufficiently severe that it seriously compromised cryoprotective perfusion. While the photo reveals only infarction in the skin, and in some cases the underlying tissues, animal studies have revealed that when this kind of vascular obstruction is observed in the skin under these conditions, it is invariably the case that the visceral organs are similarly affected.
Figure 2: The red arrows in the photo above point to areas of completely failed cryoprotective perfusion in the patient’s skin and peripheral tissues. The cause of these infarcts is post-arrest red blood cell (RBC) aggregation and micro- and macrovascular clotting secondary to prolonged warm and cold ischemia. While this patient received prompt post-arrest manual cardiopulmonary support (CPS), anticoagulation (50,000 IU sodium heparin) and external cooling (ice packs) these efforts were insufficient to prevent global vascular obstruction sufficiently severe that it seriously compromised cryoprotective perfusion. While the photo reveals only infarction in the skin, and in some cases the underlying tissues, animal studies have revealed that when this kind of vascular obstruction is observed in the skin under these conditions, it is invariably the case that the visceral organs are similarly affected.
Contemporary Human Cryopreservation Organizations (HCOs) can do little to address the last three obstacles to recovery listed above, since advances in these areas sufficient to allow recovery of today’s human cryopreservation patients, will require many decades, involve a vast interdisciplinary effort, and cost many billions or even trillions of dollars. Maturation of the enabling technologies required to implement nanoscale repair of cellular injury and tissue engineering and rejuvenation, will require the efforts and resources of the global biomedical community well into the foreseeable future, and perhaps beyond.
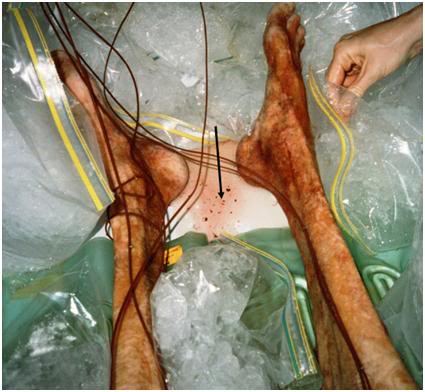 Figure 3: The black arrow points to multiple emboli in a pool of cryoprotective perfusate effluent which seeped from the sternotomy wound during perfusion of this patient. These emboli consisted of aggregates of red cells ranging in size from ~10-20 μ to ~ 3-5 mm in diameter, as well as blood clots in a range of sizes. This type of injury is largely preventable if patients receive effective cardiopulmonary support (CPS) and appropriate anticoagulation. Peri-arrest prophylactic medication with clopidrogel, combined with other agents, offers the prospect of completely inhibiting this kind of vascular obstruction in almost all human cryopreservation patients.
Figure 3: The black arrow points to multiple emboli in a pool of cryoprotective perfusate effluent which seeped from the sternotomy wound during perfusion of this patient. These emboli consisted of aggregates of red cells ranging in size from ~10-20 μ to ~ 3-5 mm in diameter, as well as blood clots in a range of sizes. This type of injury is largely preventable if patients receive effective cardiopulmonary support (CPS) and appropriate anticoagulation. Peri-arrest prophylactic medication with clopidrogel, combined with other agents, offers the prospect of completely inhibiting this kind of vascular obstruction in almost all human cryopreservation patients.
The implication of this is that where HCOs can make the most difference for the least expenditure of resources, is to focus their efforts on minimizing (and ultimately eliminating), the damage their patients suffer from IRI, and from CPA perfusion and cooling to storage temperature (currently -196 ºC). While these may seem three discrete and unrelated problems, they are, in fact, powerfully related. IRI causes vascular obstruction which impedes, or in some cases prevents adequate cryoprotection of the patient’s tissues (see Figures 2 & 3, above), resulting in greatly increased cryoinjury during subsequent deep cooling. Infarcted areas may also lead to serious exacerbation of the osmotic injury attendant to cryoprotectant loading, and the biochemical damage inflicted by IRI may exacerbate cryoprotectant toxicity, and make injured cells more susceptible to further ultrastructural degradation, or even lysis.
IRI occurs in both the peri-arrest (Standby) and post-arrest (Transport) phases of cryopreservation. These are two parts of the cryopreservation process that HCOs have the most control over, and the minimization or elimination of IRI is the core goal of Standby, Transport and pre-solidification stabilization operations. The conventional medical and biomedical context in which IRI is being researched are the areas of global cerebral ischemia and reperfusion injury (GCIRI: principally resuscitation from cardiac arrest), and regional cerebral ischemia-reperfusion injury due to myocardial infarction and stroke (RCIRI). Since these are morbid states that are of enormous public health concern, billions of dollars have been spent on developing a better understanding of the underlying pathophysiological processes in GCIRI and RCIRI, as well as on ways to treat them. Since the brain is exquisitely susceptible to both kinds of ischemia (global and regional) extensive research, both public and private, is currently underway to understand their mechanics and develop effective treatments.
It is within this context of mainstream biomedical efforts to reduce IRI injury in medicine that efforts within the human cryopreservation research establishment are best understood. This understanding can come only with a solid foundation of knowledge about the history (and context) of IRI in mainstream medicine, attempts to prevent it or treat it, and the emerging understanding of the pathophysiology of IRI. Much of the rest of this article, and subsequent ones, will be spent on providing education about the history of CPR, the pharmacological interventions being developed to treat it, and the use and effects of hypothermia on systemic IRI, primarily when used as a treatment for GCIRI, secondary to sudden cardiac arrest (SCA)[1]). Much of this discussion will take place not just in the context of human cryopreservation, but in the broader context of resuscitation medicine and public health. This perspective is being used here because the author believes it is important to understand the magnitude of the public health problem IRI constitutes, and the current focus and likely direction of research in this area – research that will hopefully, if paid attention to, have a direct and positive effect on human cryopreservation Transport operations in the future.
Finally, while few, if any, in contemporary medicine would admit it, there is reason for reciprocity between conventional medical research into disease-specific treatments for IRI, and human cryopreservation research and the development of drugs, procedures and devices aimed at minimizing IRI in human cryopreservation patients. Both spheres of activity necessarily rely on closed chest cardiac compression and mechanical ventilation (i.e., CPR and CPS) to minimize or halt ischemic damage as a result of cardiac arrest, and on non-invasive or minimally invasive methods of cooling to induce hypothermia. Consequently, clinicians and researchers in both as areas are working to improve the efficacy of CPR/CPS and to find improved ways to rapidly induce hypothermia. Similarly, the development of effective pharmacological interventions is a critically important goal of research in both human cryopreservation, and in conventional medicine in the setting of cardiac arrest and stroke.
RCIRI as a result of stroke, and GCIRI secondary to cardiac arrest, both share many elements of the same pathophysiology. As a consequence, pharmacological intervention to prevent, reverse or at least reduce this injury, also share a great deal of commonality. Advances in research to improve the medical management of stroke and GCIRI have historically served as the foundation upon which human cryopreservation Transport protocols and technology have been built. If human cryopreservation research into these areas expands in scope and quality, it seems likely that insights gained, and technologies developed in improving the care of human cryopreservation patients, may be of value in mainstream medicine as well.
For these reasons, this article has been written to address both spheres of endeavor; Transport of the human cryopreservation patient with its attendant peri- and post arrest ischemia, and treatment by mainstream medicine of patients suffering from stroke and cardiac arrest. The use of strategies to deal with GIRI will be discussed, not only in the context of human cryopreservation Transport and stabilization operations, but in the context of the medical benefits that may accrue to the community of IRI patients as a whole.
Closed Chest Cardiopulmonary Resuscitation/Support (CC-CPR/S): Limits and Promise
In 1960 Kouwenhoven, Jude, and Knickerbocker reported the use of closed-chest cardiopulmonary resuscitation (CC-CPR) in 20 patients with a 70% overall survival rate. [1] In the decades that followed, an international program of enormous scope and cost was launched to implement CC-CPR at every level of emergency care, including the instruction of millions of laypersons in the technique.
In the intervening three decades since CC-CPR was first introduced, with the enthusiastic statement by Kouwenhoven, et al., that, “Anyone, anywhere, can now initiate cardiac resuscitation procedures. All that is needed are two hands,” [2] many studies have been published documenting its ineffectiveness (i.e., survival rates, at best, under 20%) in maintaining cerebral viability in cases of cardiac arrest both in hospital [3,4] and in the field. [5,6,7] Indeed, there is evidence that the survival rate of patients experiencing in-hospital cardiac arrest has declined since CC-CPR replaced open chest CPR (OC-CPR) in the 1960′s.8 In the thirty years since its implementation there has never been a formal, organized assessment of the utility of CC-CPR in terms of cost vs. benefit, either financially or medically.
In patients who survive following resuscitation with CC-CPR, the incidence of both transient and permanent neurological deficits and reduced quality of life are high. [9-12]
In recent years there has been a growing awareness of the inadequacy of CC-CPR, with a call by some to return to OC-CPR13 and vigorous research by others to optimize CC-CPR to address the dismal survival rates and usually poor neurological outcome. Increasingly, public healthcare policy is coming to reflect the reality that neurologists, cardiologists and intensivists have long understood: “CC-CPR doesn’t work.” This is reflected in the recent policy change by the American Red Cross, wherein bystanders are now instructed to call for help and activate the Emergency Medical System (EMS) first, and start CPR second, instead of the other way around. [13] This change reflects a growing awareness that CC-CPR is largely ineffective and that a patient’s best chance for recovery is early defibrillation and associated definitive therapy (see Figures 4 & 5, below). [14]
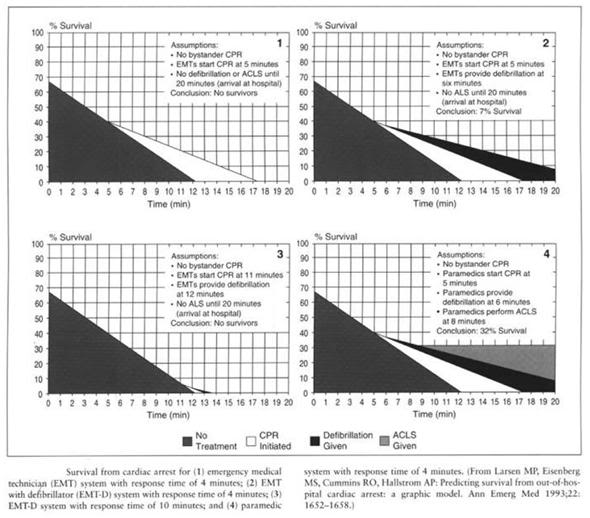 Figure 4: Dismal rate of survival after cardiac arrest.
Figure 4: Dismal rate of survival after cardiac arrest.
This may seem an extreme statement, particularly to those who have not witnessed the all too common tableaux played out in intensive care units around the world of the brain dead, or vegetative cardiac arrest victim, consuming tens or even hundreds of thousands of dollars in medical resources.
The staggering cost of CC-CPR in teaching, health care, and patient/family emotional and financial resources, when weighed against the dubious benefits, suggests that society might have been better served if the CC-CPR program had never been implemented. Thus, the conclusion seems inescapable that CC-CPR is most effective at producing individuals who either are brain dead, or in a persistent vegetative state.
The problem with CC-CPR (or any in-field resuscitation technique) is primarily cerebral ischemia-reperfusion injury (CIRI) since the brain is selectively vulnerable to and usually fails to recover from ischemic intervals much in excess of 4-6 minutes. While mechanical, or other device-oriented means of optimizing CC-CPR may well be developed, and the first-response use of defibrillators may become more commonplace, the fundamental problem of ischemic time before restoration of adequate circulation remains.
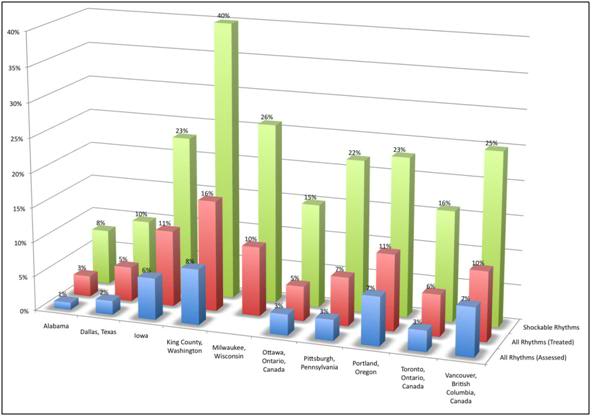 Figure 5: Survival in various US cities following sudden cardiac arrest (SCA) and CPR. Survival is consistently (and not surprisingly) greatest in those patients successfully defibrillated within a few minutes of cardiac arrest. Mortality in other cohorts is primarily from ischemia-reperfusion injury or failure to achieve return of spontaneous circulation (ROSC).
Figure 5: Survival in various US cities following sudden cardiac arrest (SCA) and CPR. Survival is consistently (and not surprisingly) greatest in those patients successfully defibrillated within a few minutes of cardiac arrest. Mortality in other cohorts is primarily from ischemia-reperfusion injury or failure to achieve return of spontaneous circulation (ROSC).
The magnitude of the problem is staggering: Each year in the United States there are 540,000 deaths from myocardial infarction (MI) [15] (with 350,000 of these deaths occurring before the patient reaches the hospital) as a result of a non-perfusing arrhythmia, principally ventricular fibrillation. [16] This mode of SCA is also responsible for the majority of the 190,000 in-hospital deaths from MI, which typically occurs within the first 24 hours following admission. [17] Especially tragic is that 50% of these deaths occur in persons ~50 years of age or less. [18] An estimated additional 20,000 incidents of SCA occur as a result of asphyxiation, drowning, electrocution, and genetic or developmental predisposition to lethal arrhythmias (i.e., Wolf-Parkinson’s White Syndrome, congenital thickening of the interventricular septum, idiopathic arrhythmic disease) and other non-atherosclerosis related causes. This latter category of SCA typically occurs in individuals whose mean age is less than 25. [19]
Premedication
For many of these 540,000 people in the United States who will experience sudden cardiac death (SCD) in the coming year, there will be little or no possibility of rescue. Cardiac arrest will occur without warning, often in situations not conducive to activation of the EMS, or will not be discovered until many hours after it has occurred. However, for many of those patients, there will have been a warning that they are at increased risk of SCA. A prior myocardial infarct (MI), familial history of arrhythmic disease, or iatrogenic risk such as CABG or angioplasty, will often provide ample warning that SCA could occur. In MI alone, the incidence of SCA resulting in death within the first year following infarct is 14%. [20] The development of more sophisticated markers for SCA in post MI patients, such as increased R-R interval regularity, is also making it possible to identify, with increasing accuracy, those who are at risk of SCA. [21] This begs the question: “What might be done to provide both neuro- and systemic protection against ischemia reperfusion injury (IRI) in this high risk population before it occurs?
Because terminally ill human cryopreservation patients are certain to experience IRI within a relatively short, predictable time frame (~6 months) it has been possible to pre-medicate them to minimize the amount of IRI they will suffer as a result of both peri-arrest shock, and post arrest IRI. While experience with this approach is very limited, it appears promising, and there can be little question but that it should be used for human cryopreservation patients as often as possible where feasible. Insights gained from this experience, especially with the use of benign and inexpensive, but nevertheless powerfully protective molecules such as melatonin and CoQ10, may have important implications in mainstream medicine for the prophylactic treatment of patients at risk for heart attack, SCA and stroke.
At this time the principal conventional medical management of SCA consists of initiation of manual, “bystander” cardiopulmonary resuscitation, so-called Basic Cardiac Life Support (BCLS), followed by “definitive” treatment of the arrhythmia, beginning with defibrillation and continuing with the application of Advanced Cardiac Life Support (ACLS), [23]
Advanced Cardiac Life Support (ACLS) and Outcome from SCA
ACLS consists of the application of an algorithm of manual CPR, electrical defibrillation and pharmacologic therapy aimed at restoring a perfusing cardiac rhythm and sufficient blood pressure and cardiac output to sustain life until definitive treatment of the underlying cause of the cardiac arrest can be achieved (e.g., coronary revascularization, implantation of an automatic defibrillator, or life-long anti-arrhythmic therapy). [24]
As is shown in Figure 4 below, the time to survival without neurological deficit following cardiac arrest in the absence of BCLS, declines rapidly, following a sigmoidal curve, with survival absent neurological deficit being ~95% following 1 minute of arrest time, and 0% following 9 minutes of arrest. [25] Put another way, 50% of patients will experience significant morbidity or death after 4 minutes of circulatory arrest.
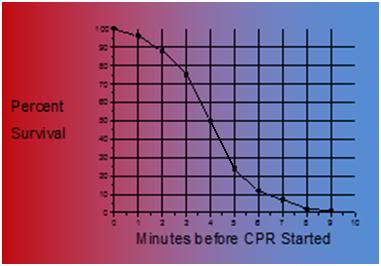 Figure 1-6: Probability of survival without severe neurological deficit as a function of time following cardiac arrest.
Figure 1-6: Probability of survival without severe neurological deficit as a function of time following cardiac arrest.
What is not shown in this table is that the effect of immediate bystander CPR on survival is negligible in most studies, [25,26] with the primary benefit being observed in patients who’s time from the initiation of BCLS, to successful cardiac resuscitation, was greater than 8 minutes.27 There is evidence in the literature that morbidity is improved with prompt by-stander CPR [28] providing that EMS response is also rapid, although this remains controversial. [24,25] A corollary of this is that the overall survival rate following SCD, with, or without, serious neurological morbidity ranges between 1% (New York City, NY) to 17% (Seattle, WA). [29] The mean survival (defined as survival to discharge from the hospital) in the United States as a whole, is generally agreed to be, at best, 15% 30 with ~70% of these patients experiencing lasting neurological morbidity (ranging from “mild” cognitive impairment to total incapacitation in the Persistent Vegetative State (PVS). [31-34]
The primary cause of non-survival in patients experiencing SCA is failed cardiac or cerebral resuscitation. Arguably, it is failed cerebral resuscitation, since most underlying causes of refractory cardiac arrest could be treated by ‘bridging’ supportive technologies, such as emergency femoral-femoral cardiopulmonary bypass (CPB), until myocardial revascularization and hemodynamic stabilization could be achieved. [35] When this technology is applied to patients who are candidates for good neurological outcome, the survival rate is increased. [36-38] These technologies are not typically used on patients who are unsuccessfully resuscitated (no restoration of adequate cardiac rhythm and perfusion), because of the justified perception that currently irreversible brain damage would have occurred, during the prolonged period of cardiac arrest or CPR/ACLS. [39] Similarly, it is for this reason that most attempts to achieve cardiopulmonary resuscitation in-hospital, in patients who are not hypothermic or intoxicated with sedative drug(s), are terminated following 15 minutes. [40,41]
It is noteworthy that both past and present ACLS protocols contain no drugs aimed at treating the primary cause of failed or morbid resuscitation from SCD: post-ischemia-reperfusion encephalopathy (American Heart Association. Advanced Cardiac Life Support. Ed. Richard O. Cummins. Dallas: American Heart Association, 2006).
21st Century Medicine’s Program of GIRI Research in a Canine Model of SCA
Over the past 15 years a vast number of therapeutic interventions have shown great promise in animal models in the laboratory. [42-45] However, none of these has been successfully applied clinically, despite numerous attempts. [46,47] These reasons include: a) the inappropriateness of many of the animal models being used to validate pharmacologic or other means of therapeutic intervention, b) failure to address the multifactorial nature of the pathophysiology of ischemia-reperfusion injury, and c) the inability to rapidly induce systemic mild therapeutic hypothermia (33oC-34oC) which, (arguably), has been shown to be one of the most potent interventions in achieving improved outcome from prolonged periods of cardiac arrest and the resulting normothermic systemic and, particularly, cerebral ischemia, they inflict.
 Figure 7: The 21CM canine resuscitation model consisted of an interval of normothermic ischemia followed by extracorporeally assisted restoration of blood circulation and induction of mild therapeutic hypothermia (MTH) using a combination hollow fiber membrane oxygenator and heat exchanger. Following CPB initiated reperfusion the animals were defibrillated as soon as possible and weaned from extracorporeal support. Induction of MTH to target temperature was achieved within 6-10 minutes of the start of CPB.
Figure 7: The 21CM canine resuscitation model consisted of an interval of normothermic ischemia followed by extracorporeally assisted restoration of blood circulation and induction of mild therapeutic hypothermia (MTH) using a combination hollow fiber membrane oxygenator and heat exchanger. Following CPB initiated reperfusion the animals were defibrillated as soon as possible and weaned from extracorporeal support. Induction of MTH to target temperature was achieved within 6-10 minutes of the start of CPB.
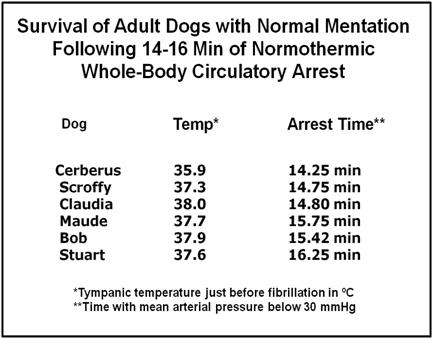
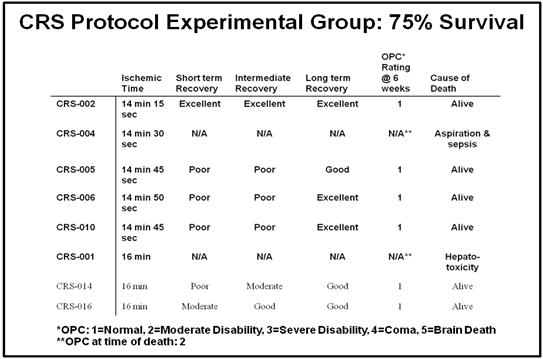 Figure 8: Outcomes in the 21CM canine resuscitation research program.
Figure 8: Outcomes in the 21CM canine resuscitation research program.
In 1994, 21st Century Medicine (21CM) of Rancho Cucamonga, CA, began a program of research to investigate the used of multimodal drug therapy, combined with mild, post resuscitative hypothermia (33◦C-34◦C). By Early 1996, 21CM was achieving routine recovery of dogs from ~16+ minutes of normothermic ischemia with 75% overall long term survival (<3 months) with ~ 75% detectable neurological deficit in the survivors. These extraordinary result were achieved by a multimodal drug protocol, using a slightly modified version of Safar, et al.’s model of cardiopulmonary bypass (CPB), with hemodilution and prompt institution of mild therapeutic hypothermia (33◦C-34◦C), to achieve initial restoration of circulation and oxygenation. [48-51]
Neurobehavioral and histological evaluation of randomly selected survivors from this study demonstrated no detectable deficits in 75% of the surviving animals (exceptions were some neuronal loss in the cerebellum, which was not associated with any demonstrable long-term disability, or motor deficit).
It is noteworthy that no other investigators have come close to demonstrating these kinds of survival times, following whole body normothermic cardiac arrest in dogs, with such low levels of neurological deficit.
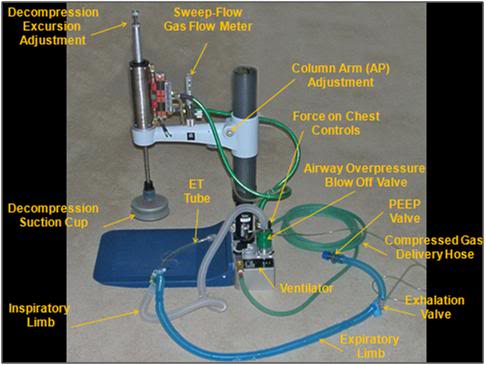 Figure 9: Active Compression-Decompression High Impulse CPR/S Device with for use with an Airway Impedance Valve
Figure 9: Active Compression-Decompression High Impulse CPR/S Device with for use with an Airway Impedance Valve
In emergency medicine, as in human cryopreservation, it is not feasible to initiate extracorporeal support within a few minutes, in cardiac arrest under field conditions, and yet the generation of adequate perfusion at a relatively high mean arterial pressure (80-90 mm Hg in the setting of MI and post arrest cardiovascular depression) is essential to survival under these conditions. The implication is clear; a method must be available which allows for the generation of adequate perfusion, at adequate MAPs, using some modality of closed chest CPR/S.
A major focus of BioPreservation, Inc., (BPI) research was the development of a heart-lung resuscitator (HLR) using closed chest CPR/S that could deliver effective perfusion in human cryopreservation patients. This device wwas developed, and demonstrated the ability to deliver prolonged periods of close chest CPS with mean arterial pressures (MAPs) which are either physiologic, or supraphysiologic, in human cryopreservation patients. It is especially noteworthy that these were patients who had experienced short intervals of cardiac arrest (legal death) preceded by long periods of peri-mortem shock, as a result of terminal cancer and end stage liver disease, and who were not candidates for ACLS and restoration of spontaneous circulation (ROSC). [52]
The device combines three technological innovations developed in clinical CPR which have not heretofore been integrated: high impulse CPR, which consists of rapid, forceful compressions with a hold time equal to ~50% of the duty cycle, active decompression of the chest using a silicone rubber suction cup on the upstroke part of the duty cycle, and a specially constructed valve which closes the airway during upstroke to maximize negative intrathoracic pressure which greatly increases venous return to the heart thus improving pre-load (effectively “priming the pump” with blood for the next compression). A more presented in a subsequent article here.
Many of the therapeutic drugs which have proven effective in the 21CM pilot study of sudden cardiac arrest (prolonged systemic ischemia) are likely to be far more effective if administered prior to the insult (see Premedication, above), rather than after a prolonged period of ischemia, and human cryopreservation patients enjoy the advantage of being able to use some of these molecules without waiting Food and Drug Administration (FDA) approval, since several are available as over the counter (OTC) nutritional supplements.
Additionally, because the time and place of their impending cardiac arrest will often be known in advance (i.e., terminal patients) a subset of human cryopreservation patients will enjoy the ability to begin protective cooling during the insult period, as opposed to having to wait for several hours before cooling can begin, let alone reach therapeutic levels, as is currently the case in mainstream medical patients today (i.e., in human cryopreservation patients external cooling can commence immediately after cardiorespiratory arrest and the pronouncement of legal death).
The 21CM protocol, with modifications, now serves as the basis for Alcor’s human cryopreservation Transport Protocol. The details of the pharmacological basis of this approach to moderating ischemia-reperfusion injury in human cryopreservation patients will be discussed in a subsequent article. The objective of the 21CM CRS program was to achieve, at minimum, the equivalent of one standard deviation of improvement in outcome following SCA and CPR (Figure 10, below). This goal was more than reached. It is hoped that application of the CRS protocol to human cryopreservation patients will significantly reduce the devastating ischemia reperfusion injury that is currently all too common. To understand the character and extent of this injury it is now necessary to explore the pathophysiology of global and regional cerebral ischemia-reperfusion injury (GCIRI & RCIRI).
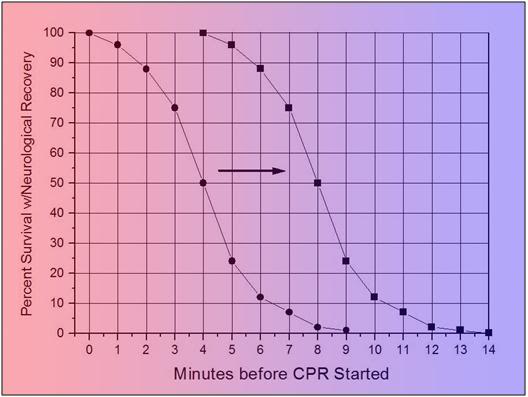 Figure 10: Graphic exposition of the effect of improving neurologically intact survival following CPR in SCA by one standard deviation.
Figure 10: Graphic exposition of the effect of improving neurologically intact survival following CPR in SCA by one standard deviation.
End of Part 1
[1] Erroneously called sudden cardiac death (SCD).
References
1) American Heart Association and National Research Council, Standards for Cardiopulmonary Resuscitation (CPR) and emergency cardiac care (ECC). J Amer Med Assoc (Suppl.). 1974;227: p. 833-68.
2) Ibid.
3) Weale, FE, Rothwell-Jackson, RL., The efficacy of cardiac massage. The Lancet. 1960. 1: p. 990-96.
4) Eisenberg, MS, Harwood, BT, Cummins, RO, Reynolds-Haertle, R, Hearne TR., Cardiac arrest and resuscitation: A tale of 29 cities. Ann of Emer Med. 1990. 19: p. 179-86.
5) Kentsch, M, Stendel, M, Berkel, H., Early prediction of prognosis in out-of-hospital cardiac arrest. Intensive Care Med. 1990. 16: p. 378-83.
6) Troiano, P, Masaryk, J, Stueven, HA, et al., The effect of bystander CPR on neurologic outcome in survivors of prehospital cardiac arrests. Resuscitation 1989;17: p. 91-98.
7) Bossaert, L, Van Hoeyweghen, R., The Cerebral Resuscitation Study Group. Resuscitation. 1989. 17m Suppl.: p. S55-S69.
8) Del Guercio, LRM, Feins, NR, Cohn, JD, et al., A comparison of blood flow during external and internal cardiac massage in man. Circulation 1965. Suppl. 1: p. 171-80.
9) Troiano, P, Masaryk, J, Stueven, HA, et al., The effect of bystander CPR on neurologic outcome in survivors of prehospital cardiac arrests. Resuscitation. 1989. 17: p. 91-98.
10) Bengtsson, M, et al., A psychiatric-psychological investigation of patients who had survived circulatory arrest. Acta Psychiat Scan. 1969. 45: p. 327.
11) Lucas, BGB., Cerebral anoxia and neurologic sequelae after cardiac arrest. In Stephenson HE, ed. Cardiac Arrest and Resuscitation, 4th Ed. St Louis:The CV Mosby Co. 1974: p. 681-707.
12) Myerburg, RJ, Conde, CA, Sung, RJ., Clinical, electrophysiologic, and hemodynamic profiles of patients resuscitated from prehospital cardiac arrest. Amer J Med. 1980. 68: p. 568.
13) Del Guercio, LRM., Open chest cardiac massage: An overview. Resuscitation.1987. 15: p. 9-11.
14) Luria, MH, Knoke, JD, Margolis, RM, et al., Acute myocardial infarction: prognosis after recovery. Ann Inter Med. 1976. 85: p. 561-63.
15) Wong, M.J, Lenihan, MJ., Advances in cardiopulmonary resuscitation. Crit Care Nurs Clin North Am. 1995. 7(2): p. 227-37.
16) de Vreede-Swagemakers, J.J., et al., Out-of-hospital cardiac arrest in the 1990′s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997. 30(6): p. 1500-5.
17) Association, A.H., American Heart association and National research Council, Standards for cardiopulmonary resuscitation (CPR) and emergency cardiac care (ECC). J Amer Med Assoc. 1974. 227 (suppl): p. 833-68.
18) Sakai, A., Sudden deaths among male employees: a six-year epidemiological survey. J Cardiol. 1990. 20(4): p. 957-61.
19) Safranek, D.J., M.S. Eisenberg, Larsen, MP., The epidemiology of cardiac arrest in young adults. Ann Emerg Med. 1992. 21(9): p. 1102-6.
20) Skogvoll, E., et al., Out-of-hospital cardiopulmonary resuscitation: a population-based Norwegian study of incidence and survival. Eur J Emerg Med. 1999. 6(4): p. 323-30.
21) Weale, F., The efficacy of cardiac massage. Lancet. 1960. 1: p. 990-96.
22) Eisenberg, M., Cardiac Arrest and Resuscitation: A tale of 29 cities. Ann of Emer Med. 1990. 19: p. 179-86.
23) Kentsch, M., et al., Early prediction of prognosis in out-of-hospital cardiac arrest. Intensive Care Med. 1990. 16(6): p. 378-83.
24) Troiano, P., et al., The effect of bystander CPR on neurologic outcome in survivors of prehospital cardiac arrests. Resuscitation. 1989. 17(1): p. 91-8.
25) Bossaert, L, Van Hoeyweghen, R., Bystander cardiopulmonary resuscitation (CPR) in out-of-hospital cardiac arrest. The Cerebral Resuscitation Study Group. Resuscitation. 1989. 17(Suppl): p. S55-69; discussion: p. S199-206.
26) Stueven, H., et al., Bystander/first responder CPR: ten years experience in a paramedic system. Ann Emerg Med. 1986. 15(6): p. 707-10.
28) Lombardi, G., J. Gallagher, P. Gennis, P., Outcome of out-of-hospital cardiac arrest in New York City. The Pre- Hospital Arrest Survival Evaluation (PHASE) Study [see comments]. JAMA. 1994. 271(9): p. 678-83.
29) McCarthy, M., Looking after your neighbors Seattle-style. Lancet. 1998. 351: p. 732. 15.
30) Bengtsson, M., A psychiatric-psychosocial investigation of patients who had survived circulatory arrest. Acta Psychiat Scan. 1969. 45: p. 327.
31) Roewer, N., T. Kloss, and Puschel, K., Long-term result and quality of life following preclinical cardiopulmonary resuscitation. Anasth Intensivther Notfallmed. 1985. 20(5): p. 244-50.
32) de Vos, R., Quality of life after cardiopulmonary resuscitation. Resuscitation. 1997. 35(3): p. 231-6.
33) Roewer, N., T. Kloss, Puschel, K., Long-term result and quality of life following preclinical cardiopulmonary resuscitation. Anasth Intensivther Notfallmed. 1985. 20(5): p. 244-50.
34) de Vos, R., Quality of life after cardiopulmonary resuscitation. Resuscitation. 1997. 35(3): p. 231-6.
35) Phillips, S.J., Resuscitation for cardiogenic shock with extracorporeal membrane oxygenation systems. Semin Thorac Cardiovasc Surg. 1994. 6(3): p. 131-5.
36) Younger, J.G., et al., Extracorporeal resuscitation of cardiac arrest [see comments]. Acad Emerg Med. 1999. 6(7): p. 700-7.
37) Matsuwaka, R., et al., Emergency percutaneous cardiopulmonary support for patients with cardiac arrest or severe cardiogenic shock. Nippon Kyobu Geka Gakkai Zasshi. 1996. 44(11): p. 2006-10.
38) Myerburg, R., Clinical, electrophysiologic, and hemodynamic profiles of patients resuscitated from pre-hospital cardiac arrest. Amer J Med. 1980. 68: p. 568.
39) Peterson, M.W., et al., Outcome after cardiopulmonary resuscitation in a medical intensive care unit. Chest. 1991. 100(1): p. 168-74.
40) Gener, J, et al., Immediate and 1-year survival after cardiopulmonary resuscitation at an intensive care unit. Med Clin (Barc). 1989. 93(12): p. 445-8.
41) Peterson, M.W, et al., Outcome after cardiopulmonary resuscitation in a medical intensive care unit. Chest. 1991. 100(1): p. 168-74.
42) Gener, J, et al., Immediate and 1-year survival after cardiopulmonary resuscitation at an intensive care unit. Med Clin (Barc). 1989. 93(12): p. 445-8.
43) Kim, H, et al., Amelioration of impaired cerebral metabolism after severe acidotic ischemia by tirilazad post-treatment in dogs. Stroke. 1996. 27(1): p. 114-21.
44) Iwatsuki, N, et al., Hyperbaric oxygen combined with nicardipine administration accelerates neurologic recovery after cerebral ischemia in a canine model. Crit Care Med. 1994. 22(5): p. 858-63.
45) Vaagenes, P, et al., Amelioration of brain damage by lidoflazine after prolonged ventricular fibrillation cardiac arrest in dogs. Crit Care Med. 1984. 12(10): p. 846-55.
46) Abiko, H, et al., Cerebral protective effect of flunarizine in a canine model of cerebral ischemia. No To Shinkei. 1987. 39(9): p. 847-54.
47) Borger, M.A, Weisel, RD., Calcium channel blockers in myocardial and cerebral ischemia: a clinician’s review from bench to bedside. Can J Cardiol. 1999. 15(3): p. 333-40.
48) Hickenbottom, S.L, Grotta, J., Neuroprotective therapy. Semin Neurol. 1998. 18(4): p. 485-92.
49) Safar, P., Cerebral resuscitation. Mt Sinai J Med. 1981. 48(4): p. 385-8.
50) Safar, P., Long-term animal outcome models for cardiopulmonary-cerebral resuscitation research. Crit Care Med. 1985. 13(11): p. 936-40.
51) Safar, P. and N.G. Bircher, Resuscitative cerebral hypothermia after cardiac arrest [letter; comment]. Crit Care Med.1994. 22(10): p. 1703-4.
52) Darwin, M., Effects of ACD-CPR and vasopressin on human cryonics patients following 1-5 min of cardiac arrest. Unpublished Results, 1996.
