By Mike Darwin
Excitotoxicity
A rapidly growing body of evidence indicates that excitatory neurotransmitters, primarily the excitatory amino acids (EEAs) aspartate, N-methyl-D-aspartate (NMDA), homocysteine and cysteine, which are released from neurons during ischemia, play an important role in the etiology of neuronal ischemic injury.112-114 Those areas of the brain which show the most “selective vulnerability” to ischemia, such as the neocortex and hippocampus, are richly endowed with excitatory AMPA (alpha-amino-hydroxy-5-methyl-4-isoxazole proprionic acid) and NMDA (N-methyl-d-aspartate) glutamate receptors.115
Glutamate is the most abundant EAA in the brain serving as a neurotransmitter, a metabolite, and a neurotrophic molecule116,117 It is compartmentalized in neurons and released to the extracellular space primarily to accomplish its role as a neurotransmitter. Under normal conditions extracellular glutamate is rapidly pumped back into the neurons. However, in ischemia and in some pathological states such as Huntington’s disease and amyotrophic lateral sclerosis (ALS), glutamate re-uptake is impaired or inhibited.
Glutamate and other EEA release begins as soon as membrane depolarization occurs, and continues throughout the first 10-15 min of GCI, until equilibrium is reached between the intra- and extracellular spaces.117 Elevated extracellular levels of glutamate open the NMDA-gated Ca++ receptor channels and increase intracellular Ca++. High extracellular levels of glutamate also interfere with cysteine uptake, resulting in the depletion of cellular glutathione; the most abundant intracellular antioxidant molecule, further exacerbating IRI.118, 119
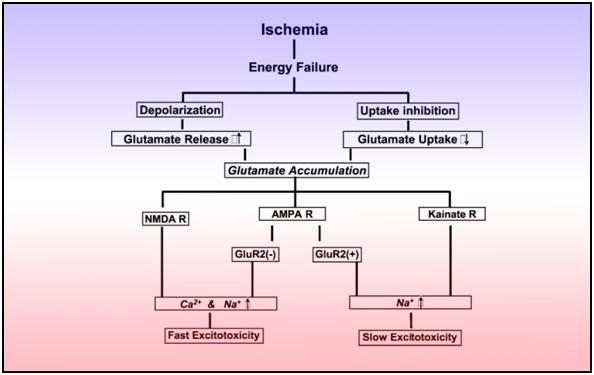 Figure 18: Two possible biochemical cascades that induce post-ischemic excitotoxicity.
Figure 18: Two possible biochemical cascades that induce post-ischemic excitotoxicity.
The primary targets of excitotoxic injury are the neuronal cell body and dendrites (possibly due their rich endowment with membrane glutamate receptors), with relative sparing of axons, glia, and ependymal and endothelial cells. Glutamate activates three major families of ionophore linked receptors (NMDA, aamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate) as well as the metabotropic receptors that activate a wide variety of second messenger systems.120 Although glutamate release concurrently activates NMDA and AMPA receptors, in vitro studies demonstrate that glutamate toxicity occurs in two distinct phases. The first occurs when excitotoxicity is rapidly induced by brief, intense stimulation of NMDA receptors as in GCIRI, which are critically dependent on the presence and influx of extracellular Ca++ through the NMDA-gated receptor channel complex. The second wave of excitotoxicity, which is commonly seen in penumbral neurons in RIRI, is generated by a slowly triggered process when there is prolonged stimulation of AMPA/kainate receptors that have limited Ca++ channels.121 Metabotropic glutamate receptors do not initiate excitotoxicity injury; instead they serve to amplify the injury once it has begun. This amplification via the slowly triggered process is also operative in GCIRI in hypo-perfused regions post ROSC.
The cascade of events responsible for glutamate excitotoxicity includes three distinct processes:
1) Induction, whereby extracellular glutamate efflux is transduced by receptors on the neuronal membrane to cause intracellular Ca++ overload, which leads to lethal intracellular derangements.
2) Amplification of the derangement, with an increase in intensity and involvement of other neurons.
3) Expression of cell death triggered by cytotoxic cascades.121
Excess release of Ca++ and its intracellular influx is thought to be the primary trigger for a variety of complex, deleterious intracellular processes that result from the activation of catabolic enzymes, such as phospholipases which lead to cell membrane breakdown, arachidonic acid, and free radical formation, and endonucleases which lead to fragmentation of genomic DNA and energy failure due to mitochondrial dysfunction.
Some subpopulations of neurons in the brain are selectively vulnerable to excitotoxic injury (and thus to ischemic injury), possibly from differences in excitatory synaptic inputs, distribution and density of glutamate receptors, and possibly, differences in intrinsic defence mechanisms, such levels of antioxidants and efficacy of DNA repair. Perhaps the best evidence that excitotoxicity plays a cardinal role in CIRI is the fact that in many models of both GCIRI and RCIRI substantial neuroprotection can be achieved with the use of NMDA or AMPA receptor antagonists.122
In addition to the NMDA receptor mediated excitotoxins, there are at least two other excitatory molecules that appear to play a role in CIRI; dopamine and norepinephrine. Both of these neurotransmitters efflux into the extracellular space beginning at the time that membrane depolarization occurs.123-126 Currently there is only indirect evidence of their role in the pathophysiology of CIRI in the form of reduced histopathological injury when the levels of these neurotransmitters are reduced using barbiturates or isoflurane.127-128 Additional experimental evidence that these, and possibly other catecholamines, may be responsible for some of the injury in CIRI is that surgical ablation of the nigrostriatal tract (thus reducing striatal dopamine content) protects intrinsic striatal neurons from injury following global cerebral ischemia.129, 130 Similarly, depletion of catecholamine stores by a-methyl-paratyrosine exerts a marked protective effect on ischemic damage to synaptic terminals.131, 132 Although the precise mechanism of neuronal injury by dopamine is not known, its metabolites include hydrogen peroxide, the superoxide ion, and hydrogen radicals; all of which have been implicated in the neuron loss in the substantia nigra that is implicated in the pathogenesis of Parkinson’s disease.
Initially there was much optimism that blockade of the NMDA receptor would provide protection against delayed neuronal death following global cerebral ischemia.133,134 However, the NMDA antagonists now clinically available have proved ineffective or too toxic in human clinical trials.135,136 The use of experimental NMDA receptor blocking drugs has shown significant promise in ameliorating focal cerebral ischemic injury in animal models of RCIRI, demonstrating a marked reduction in the severity of ischemic injury in the poorly perfused “penumbra” surrounding the no-flow area of the infarct.137 Furthermore, in vitro studies with cultured neurons have demonstrated that excitatory neurotransmitters cause neuronal injury and death, even in the absence of hypoxic or ischemic injury.138
In RCIRI, the NMDA receptor remains activated for a long period due to the prolonged interval of poor perfusion in the penumbra. However, in GCIRI there is good resumption of blood flow following restoration of circulation, with prompt uptake of glutamate and aspartate, and rapid inactivation of the NMDA receptors.139 Since acidosis is known to inactivate the NMDA receptor, another factor limiting the role of the NMDA receptor in mediating injury in GCIRI may be the rapid and pronounced drop in pH which occurs in neurons in global (as opposed to focal) ischemia. These are probably the reasons why NMDA receptor inhibitors have not proved effective in preventing GCIRI.140 Recently, attention has turned to non-NMDA receptor antagonists such as inhibitors of the kainate and AMPA receptors.141, 142 However, it is too early to tell if inhibition of these receptors will prove a viable therapeutic target.
While the mechanisms whereby excitotoxins cause cell injury are not yet fully understood, it is known that they facilitate calcium entry into neurons143-145 and that they are directly cytotoxic in high enough concentrations. They are neurotoxic, even in cell culture where the medium is calcium free.146 In the case of kainate and AMPA receptor activation, the likely mode of injury is sensitization of the CA1 pyramidal cells during ischemia, such that when normal signaling is restored at the end of the ischemic insult, and normal intensity input from the Schaffer collaterals is resumed, lethal cell injury results, perhaps from abnormal calcium regulation in the CA1 cells.
Mitochondrial Dysfunction
Research that has taken place over the past decade has made it abundantly clear that the mitochondria are the motors of injury in IRI; they are both the target and cause of an enormous amount of reperfusion injury.147-149 While it has been understood since at least the 1ate 1960s that Ca++ loading of the mitochondria occurs during both ischemia and reperfusion, as evidenced by flocculent densities (consisting of Ca++ crystals) seen with transmission electron microscopy in the mitochondria of IRI injured hearts. However, it is only very recently that the mechanics of how Ca++ overload disrupts mitochondrial function and structure have begun to be elucidated.
If the mitochondria are the motors of IRI, then the mitochondrial permeability transition pores ((PT) pores) are the pistons in those engines The (PT) pore is a nonselective, high conductance channel in the mitochondrial membrane which normally remains closed, or briefly flickers open in response to mitochondrial Ca++ cycling. It appears to be composed of three macromolecules of widely divergent character; the VDAC (voltage-dependent anion channel), ANT (adenine nucleotide translocator) and CypD (cyclophilin D).150 The (PT) pore appears to exist only at junctions between the inner and outer mitochondrial membranes.151 High levels of Ca++ within mitochondria under the conditions of ischemia-reperfusion cause the (PT) pore to open.152 This may be because Ca++ binds to and activates Ca++ binding sites on the matrix side of the (PT) pore.153,154 (PT) pore induction is also induced as a result of the collapse of the voltage differential between the inside and outside of mitochondrial membranes (permeability transition, or δψ).155 Additionally, free radicals generated as a result of the interference of calcium with electron transport may also directly open the (PT) pore. The (PT) pore remains closed during the ischemic interval because it is inhibited in acidosis and the intracellular milieu during ischemia is profoundly acidotic.156 The pore is also inhibited by elevated concentrations of ADP156 ATP, 157 and NADH.158 Magnesium (Mg+) and other divalent cations also inhibit the (PT) pore, because they can compete with Ca++ for the Ca++ binding sites on the matrix side of the (PT) pore.159
Induction of the (PT) pore further increases mitochondrial membrane permeability, which in turn causes the mitochondria to become further depolarized, resulting in the abolition of Δψ. When Δψ is lost, protons and molecular species up to 1.5 kd are able to flow freely across the outer mitochondrial membrane160,161 and adenosine triphosphate (ATP) production is compromised due to the absence of the electrochemical gradient across the mitochondrial membrane that is required to drive ATP production.
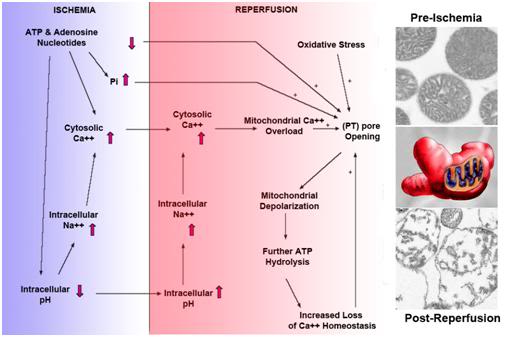 Figure 19: The biochemical cascade involved the opening of the (PT) pore in both regional and global cerebral ischemia. At left are shown normal (pre-ischemic) mitochondria and ruptured post-ischemic mitochondria. The drawing, center left, illustrates the current view of the mitochondrion obtained from single-photon confocal imaging as an irregular, heterogeneously shaped organelle, in contrast to the previous view that depicted a uniform sausage shape. The latter was apparently an artifact of fixation and the ability to view the mitochondrion only in ultrathin cross-section.
Figure 19: The biochemical cascade involved the opening of the (PT) pore in both regional and global cerebral ischemia. At left are shown normal (pre-ischemic) mitochondria and ruptured post-ischemic mitochondria. The drawing, center left, illustrates the current view of the mitochondrion obtained from single-photon confocal imaging as an irregular, heterogeneously shaped organelle, in contrast to the previous view that depicted a uniform sausage shape. The latter was apparently an artifact of fixation and the ability to view the mitochondrion only in ultrathin cross-section.
With the (PT) pore open, critical intra-mitochondrial metabolites, antioxidants (such as glutathione), and tricarboxylic-acid-cycle intermediates are lost to the cytosol. ATP is hydrolyzed and small electron transport proteins, most notably cytochrome C , diffuse out of the mitochondria. Under these conditions, ATP synthase may be activated and the mitochondria may begin hydrolysing, rather than synthesizing, ATP. ATP hydrolysis results in further impairment of high energy metabolism, resulting in further Ca++ deregulation, further (PT) pore opening, which continues in a vicious cycle; the result being decreasing phosphorylation potential. If this hypothesis is correct, (PT) pore opening is the critical determiner of when mitochondrial injury becomes irreversible.162, 163 (PT) pore opening also allows osmotically active solutes from the cytosol to enter the mitochondria, resulting in mitochondrial swelling ending in rupture of the outer mitochondrial membrane and the release of cytochrome C (as noted previously, cytochrome C is a trigger for apoptosis.)
Since it appears the opening of the (PT) pore is the primary cause of necrotic cell death in IRI, the discovery that the immunosuppressant, anti-rejection drug cyclosporine A, a cyclic a cyclic polypeptide (11 amino acids)164, 165 and to a lesser extent the immunosuppressant macrolide tacrolimus,166 are effective at keeping the (PT) pore closed and in closing it once it is opened (even under conditions of RCIRI and GCIRI) is of potentially great therapeutic importance. While neither drug crosses the BBB in the dose range used to treat rejection, cyclosporine A (CsA) does cross the BBB in a dose-dependent manner at high doses167 and has been shown to be effective at reducing infarct size in a mouse model of RCIRI.168 While the systemic dose of CsA required to deliver adequate concentrations across the BBB is not tolerable in a conventional clinical setting due to nephrotoxicity, it may be acceptable in the setting of human cryopreservation patient Transport, where acute renal injury from drug toxicity is not a consideration. A modified version of CsA is under development for clinical use, and consists of CsA delivered in micellized form, using an emulsification system consisting of mono and di-triglycerides, polyoxyl 40 hydrogenated castor oil NF (Cremaphor™), DL-α tocopherol and propylene glycol. This product should have greatly enhanced permeability to the BBB and there is preliminary evidence, in the form of greatly increased CsA induced central nervous system (CNS) toxicity with this preparation, that it is, in fact, crossing the BBB. 169
There is also an accumulation of free fatty acids, long-chain acyl-CoA, and long-chain carnitines in both ischemic and reperfused mitochondria.170, 171 Of these alterations, the accumulation of long-chain acyl-CoA is perhaps most significant, since intra-mitochondrial accumulation of this molecule is known to be deleterious to a host of mitochondrial enzyme systems resulting in impaired ATP production.172
End of Part 3
References
115) Sommer B, Seeburg PH., Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992. 13: p. 291–6.
116) Choi, DW., Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988. 1: p. 623–34.
117) Fonnum, F., Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984. 42: p. 1–11.
117) Olney, JW., Brain lesion, obesity and other disturbances in mice treated with monosodium glutamate. Science. 1969. 164: p. 719–21.
118) Choi, DW., Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988. 1: p. 623–34.
119) Benveniste, H, Drejer, J, Schoushoe, A., et al. Elevation of extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984. 43: p. 1369–74.
120) Sommer, B, Seeburg, PH., Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992. 13: p. 291–6.
121) Choi, DW., Excitotoxic cell death. J Neurobiol. 1992. 23: p. 1261–76.
122) Choi, DW., Methods of antagonizing glutamate neurotoxicity. Cerebrovasc Brain Metab. Rev. 1990. 2: p. 105–47.
123) Bhardwaj A, Brannan T, Martinez-Tica J, et al., Ischemia in the dorsal hippocampus is associated with acute release of dopamine and norepinephrine. J Neural Transm. 1990. 80: p. 195–201.
124) Bhardwaj, A, Brannan, T, Weinberger, J., Pentobarbital inhibits extracellular release of dopamine in the ischemic striatum. J Neural Transm. 1990. 82: p. 111–7.
125) Slivka, A, Brannan, TS, Weinberger J, et al., Increase in extracellular dopamine in the straitum during cerebral ischemia: a study utilizing cerebral microdialysis. J Neurochem. 1988. 50: p. 1714–8.
126) Globus MY-T, Busto R, Dietrich WD, et al., Intraischemic extracellular release of dopamine and glutamate is associated with striatal vulnerability to ischemia. Neurosci Lett. 1988. 91: p. 36–40.
127) Koorn R, Brannan TS, Martinez-Tica J, et al. Effect of etomidate on in vivo ischemiainduced dopamine release in the corpus striatum of the rat: a study using cerebral microdialysis. Anesth Analg. 1994. 78: p. 73–9.
128) Koorn R, Kahn RA, Martinez-Tica J, et al., Effect of isoflurane and halothane on in vivo ischemia-induced dopamine release in the corpus striatum of the rat: a study utilizing cerebral microdialysis. Anesthesiology. 1993.79: p. 827–35.
129) Weinberger, J, Cohen, G, Nieves-Rosa, J., Nerve terminal damage in cerebral ischemia: greater susceptibility of catecholamine nerve terminals relative to serotonin nerve terminals. Stroke. 1983. 14: p. 986–9.
130) Weinberger, J, Nieves-Rosa, J, Cohen, G., Nerve terminal damage in cerebral ischemia: protective effect of alpha-methyl-para-tyrosine. Stroke. 1985. 16: p. 864–70.
131) Globus, MY-T, Ginsberg, MD, Dietrich, WD, et al., Subsantia nigra lesion protects against ischemic damage in the striatum. Neurosci Lett. 1987. 80: p. 251–6.
132) Kahn, RA, Weinberger, J, Brannan, T, et al., Nitric oxide modulates dopamine release during global temporary cerebral ischemia. Anesth Analg. 1995. 80: p. 1116–21.
133) Benveniste, H., Calcium accumulation by glutamate receptor activation is involved in hippocampal cell damage after ischemia. Acta Neurol Scand. 1988. 78: p. 528-36.
134) Ozyurt, E., Protective effect of glutamate antagonist MK-801 in focal cerebral ischemia in the cat. J Cereb Blood Flow Metab. 1988. 8: p. 138-43.
135) Olney, JW, Labruyere, J, Price, MT., Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989. 244 (4910): p. 1360–2.
136) Albers, Atkinson, GW, Kelley; RP, Rosenbaum, RE., Safety, tolerability, and pharmacokinetics of the N-Methyl-D-Aspartate antagonist dextrorphan in patients with acute stroke. Stroke. 1995. 26: p. 254-258.
137) Hagberg, H., Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cerebr Blood Flow Metab. 1985. 5: p. 413-19.
138) Rothman, S., Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J Neurosci. 1984. 4(7): p. 1884-91.
139)Diemer, N., N-methyl-d-aspartate and non n-d-methyl-d-aspartate antagonists in global cerebral ischemia. Stroke. 1990. Supplement III, 21: p. 39-41.
140) Buchan, AM., et al., AMPA antagonists: do they hold more promise for clinical stroke trials than NMDA antagonists? Stroke. 1993. 24(12 Suppl): p. I148-52.
141) Nellgard, B, Wieloch, T., Postischemic blockade of AMPA but not NMDA receptors mitigates neuronal damage in the rat brain following transient severe cerebral ischemia. J Cereb Blood Flow Metab. 1992. 12(1): p. 2-11.
142) Arias, RL, Tasse, JR, Bowlby, MR., Neuroprotective interaction effects of NMDA and AMPA receptor antagonists in an in vitro model of cerebral ischemia. Brain Res. 1999. 816(2): p. 299-308.
143) Kempski, OS., Neuroprotection. Models and basic principles. Anaesthesist. 1994. 43 Suppl 2: p. S25-33.
144) Schurr, A., et al., Hypoxia, excitotoxicity, and neuroprotection in the hippocampal slice preparation. J Neurosci Methods. 1995. 59(1): p. 129-38.
145) Valli, M., [Influence of excitatory amino acids on the outcome of cerebral ischemia]. Presse Med, 1987. 16(23): p. 1118-21.
146) Rothman, SM. Olney, JW., Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol. 1986. 19(2): p. 105-11.
147) Ichas, F and Mazat, JP., From calcium signalling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low to high conductance state. Biochimica et Biophysica Acta. 1998. 1366(1–2): p 33–50.
148) Schinder, AF, Olson, EC., Spitzer, NC, Montal M., Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. Journal of Neuroscience. 1996. 16(19): p. 6125-6133.
149) White, RJ, Reynolds, IJ., Mitochondrial depolarization in glutamate-stimulated neurons: An early signal specific to excitotoxin exposure. Journal of Neuroscience. 1996 16(18):, p. 5688–5697.
150) Haworth, RA, Hunter, DR., The Ca2+-induced membrane transition in mitochondria II. Nature of the Ca2+ trigger site. Archives of Biochemistry and Biophysics. 1979. 195(2): p. 460-467.
152) Hunter, DR, Haworth, RA., The Ca2+-induced membrane transition in mitochondria I. The protective mechanisms. Archives of Biochemistry and Biophysics, 1979. 195(2): p. 453-459.
153) Haworth, RA, Hunter DR., The Ca2+induced membrane transition in mitochondria II. Nature of the Ca2+ trigger site. Archives of Biochemistry and Biophysics, 1979. 195(2): p. 460-467.
154) Ichas, F and Mazat, JP., From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high- conductance state. Biochimica et Biophysica Acta. 1998. 1366(1-2): p. 33-50
155) Schinder, AF, Olson, EC, Spitzer, NC, Montal, M., Mitochondrial dysfunction is a primary event in glutamate neurotoxity. Journal of Neuroscience. 1996. 16(19): p. 6125-6133.
156) Hunter, DR, Haworth, RA., The Ca2+-induced membrane transition in mitochondria. Transitional Ca2+ release. 1979. Archives of Biochemistry and Biophysics. 195(2): p.468-477.
158) Hunter, DA, Haworth, RA., The Ca2+-induced membrane transition in mitochondria I. The protective mechanisms. 1979. Archives of Biochemistry and Biophysics. 195(2): p. 453-459.
157) Beutner, G, Rück, A, Riede, B, Brdiczka, D., Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochimica et Biophysica Acta 1998. 1368(1): p. 7-18.
158) Haworth, RA, Hunter DR., The Ca2+-induced membrane transition in mitochondria II. Nature of the Ca2+ trigger site. 1979. Archives of Biochemistry and Biophysics. 195(2): p. 460-467.
159) Haworth RA and Hunter DR., The Ca2+-induced membrane transition in mitochondria II. Nature of the Ca2+ trigger site. 1979. Archives of Biochemistry and Biophysics. 195(2): p. 460-467
160) Schinder, AF, Olson, EC, Spitzer, NC, Montal, M., 1996. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. Journal of Neuroscience. 16(19): p. 6125-6133
161) White, RJ, Reynolds IJ., Mitochondrial depolarization in glutamate-stimulated neurons: An early signal specific to excitotoxin exposure. Journal of Neuroscience. 1996. 16(18): p. 5688–5697.
162) Crompton, M. and Costi, A., A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. 1988 Eur. J. Biochem. 178, p. 489-501.
163) Crompton, M., Ellinger, H. and Costi, A., Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. 1988. Biochem. J. 255, p. 357-360.
164) Crompton, M., Kunzi, M. & Carafoli, E., Regulation of Ca2+ Efflux from Kidney and Liver Mitochondria by Unsaturated Fatty Acids and Na+ Ions.1977. Eur. J. Biochem. 79, p. 549–558.
165) Griffiths, J, Halstrap, P., Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerise Implications for the immunosuppressive and toxic effects of cyclosporine. Biochem. J. 1991. 274, p. 611-614.
166) Yokota, S, Saito, M, Ozawa, H, Nishinaka, T, Sugiyama, Y., Protective effect of FK506 on ischemia/reperfusion-induced myocardial damage in canine heart. J. 1993.Cardiovasc Pharmacol. 21(3): p. 448-54.
167) Lemaire, M, Bruelisauer, A, Guntz, P, Sato, H., Dose-dependent brain penetration of SDZ PSC 833, a novel multidrug resistance-reversing cyclosporin, in rats. Cancer. Chemother Pharmacol. 1996. 38(5):481-6.
168) Yoshimoto, T, Siesjö, BK., Posttreatment with the immunosuppressant cyclosporin A in transient focal ischemia. Brain Res. 1999. 839(2): p. 283-91.
169) Personal Communication with Merck, 11 April, 1998.
170) Strosznajder, J, et al., Metabolism of oleoyl-CoA in rat brain synaptosomes: effects of calcium and post-decapitative ischemia. Neurochem Res. 1981. 6(11): p. 1231-40.
171) Frenkel, R., Carnitine Biosynthesis, Metabolism and Functions. Carnitine Biosynthesis, Metabolism and Functions, 1980. New York, Academic Press: p. 321-340.
172) Takeuchi, Y., et al., A possible mechanism of mitochondrial dysfunction during cerebral ischemia: inhibition of mitochondrial respiration activity by arachidonic acid. Arch Biochem Biophys. 1991. 289(1): p. 33-8.
