By Mike Darwin
 Figure 1: The ultra-compact, lightweight and fully self contained Lifebridge B2T® Portable Extracorporeal Life Support System.
Figure 1: The ultra-compact, lightweight and fully self contained Lifebridge B2T® Portable Extracorporeal Life Support System.
Introduction
The Medizintechnik GmbH Lifebridge B2T® extracorporeal life support system (Figure 1) is a device fast closing in on a technology I’ve long dreamed about, and even made some tentative efforts towards developing, namely, a compact, self-contained, field-able, semi-automated cardiopulmonary bypass system.1,2 It is a beautifully engineered and elegant little machine, and I believe that it represents (at least for Europe) the beginning of a transition of extracorporeal circulatory support (ECCS) from exclusive use in the operating theatre and ICU in the hands of perfusionists, to the hands of critical care and emergency medicine physicians and nurses in the Emergency Department (ED), in the Cardiac Catheterization Lab, and ultimately, in the field. It is also the latest extracorporeal technological tour de force from Germany, making that nation the world’s undisputed leader in innovative and brilliantly engineered ECCS products. As a useful aside, I would note that the first no-pump extracorporeal ventilator, the Novalung® iLA Membrane Ventilator (Figure 2) is also a product of German extracorporeal engineering.4-7
 Figure 2: The Novalung® iLA GmbH Membrane Ventilator: no pump, no muss, no fuss.
Figure 2: The Novalung® iLA GmbH Membrane Ventilator: no pump, no muss, no fuss.
When I first saw this system undergoing animal trials in Europe ~ 2 years ago, I was deeply conflicted about reviewing it anywhere that cryonics organization personnel might see my post. Historically, there has been this thirst for the “big technological fix” in cryonics, and I could see in my mind’s eye the cash changing hands in the eager hope that the Lifebridge B2T® would make bypass foolproof and easy. No such system exists, yet. Nevertheless, the Lifebridge B2T® is unquestionably a big step along the way. The device contains a PC with sufficiently sophisticated software to allow the system to pretty much prime itself! But, I’m getting ahead of myself.
System Configuration and Performance
The unit consists of three proprietary integrated units which supply every hardware and disposable component needed for emergency cardiopulmonary bypass. The entire extracorporeal circuit is contained within an injection molded polypropylene housing that snaps easily into place. The Patient Module is incredibly rugged, and it can actually tolerate being flung on the floor with considerable force without damage to the bypass circuit components inside – although this practice is clearly not recommended. [When this was demonstrated for me it reminded me of when plastic IV bags were introduced as an alternative to glass bottles. Baxter sales reps stood on them, stomped on them, and flung them off multistory buildings!]
The components embedded in the disposable “Patient Module” consist of a 400 mL hard shell venous reservoir which feeds a clone of the Biomedicus BP80 centrifugal blood pump from which blood is delivered to the oxygenator/heat exchanger and arterial filter. The Lifebridge B2T® was originally marketed with only the Minintech BioCor 200 Polypropylene pseudomembrane oxygenator as an option, but Medizintechnik received 510(k) clearance in December of last year to begin offering the device with the Medtronic Affinity® NT oxygenator, with either Carmeda® Bioactive Surface coating, or Trillium Biopassive Surface coating. This makes the entire system completely “heparin bonded” and “blood compatible,” permitting minimal anticoagulation. The arterial filter is a Terumo Pall AL8 40 40-micron filter with an integral bypass loop. Also molded into cassette that holds the extracorporeal circuit are disposable sensors for monitoring arterial and venous pressure (including filter back-pressure), as well as sensors air for detection, and venous reservoir level monitoring and control.3
 Figure 3: The disposable, integrated Patient Module which contains the entire extracorporeal system.
Figure 3: The disposable, integrated Patient Module which contains the entire extracorporeal system.
There is a small, integral (also disposable!) roller pump head in the patient module that semi-automatically primes the extracorporeal circuit with lactated Ringer’s solution, and assists in ensuring the system is de-bubbled and ready to connect to the patient (Figure 5). This little positive displacement pump is also used to control volume in the venous reservoir. Fluid waste is dumped to a waste reservoir which is also contained within the patient module cartridge and is vented to atmosphere via a 0.45 micron hydrophobic filter to prevent aerosol contamination of the ambient air. The extracorporeal system is closed to air – there is no blood-air interface in the system, and this further reduces trauma to the blood (hemolysis and protein denaturation) and provides added inherent safety against air embolism.
The second element of the system is the Control Module, which houses the drive system, (the blood pump motor/controller, the drive motor for the roller pump active air elimination system, the automatic clamps for blood flow regulation, and an integrated back-up Lithium Polymer (LiPO) battery that allows standalone operation of the control and patient module. The entire system, including the transport case “suitcase” that the unit can be fielded in weighs ~20 kg. The (required) 1 liter of Ringer’s solution prime is not included in the system’s weight. Minus the carrying case, the system comes in at a weight of 18 kg (39.6 lb) – very impressive considering that the device subsumes a conventional extracorporeal membrane oxygenation (ECMO) cart and part of a perfusionist. Occluding clamps and all other instrumentation needed to safely operate the system are either integral to the device, or included within its transport case. The extracorporeal cartridge can safely remain in the system for 1-year after it is emplaced – further reducing the time from deployment to fully operational status. I was able to go from dry to primed in 6 minutes, and the manufacturer says it is easily possible to do so in 5. I believe them. The modular configuration and semi-automatic priming of the system allows for what is being termed “plug-and-play” initiation of ECC within 5 minutes.1
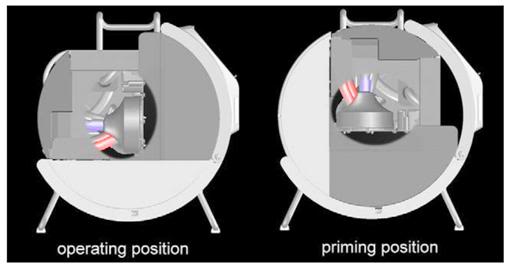
Figure 4: The semi-automatic priming procedure requires the pump head to be manually rotated 90 degrees so that the pump inlet is pointing up (at left, above). Once the circuit is primed, the pump and circuit are rotated in the operating position shown at left in the schematic above..Centrifugal pumps are inherently incapable of pumping macro-air because they de-prime when large amounts of air enters the pump head. The Lifebridge B2T® has cleverly built on this inherent safety feature by mounting the centrifugal pump head with its tangential outlet pointing down.1
The third component of the system is a base module with a tubular tilting frame that allows the machine to be rocked back 90 degrees to permit priming. That’s the semi-automatic part of the priming process – although the unit walks you through it a step at the time (Figure 4). The base module is surprisingly well designed and is quite stable – the locking mechanism is intuitive, and the tubular framework is rugged, so it is unlikely to become bent or distorted from being lugged around or transported in vehicles or aircraft. The base module houses the transformer and control electronics for charging the Bullith batteries,[1] and the unit will accept either 110 or 220 VAC current. The Bullith batteries are, again, an example of where German engineering and innovation are taking the lead – they are compact, featherweight by comparison to gel cells, they hold a lot of energy for their size and weight – and nothing else on the market comes close to their reliability and performance. They also have a fantastic shelf life and do not require continuous trickle charging to keep them from spoiling, as do NiCad and NiMh cells. That means you can leave the unit on the shelf, much like an Automatic External Defibrillator (AED), until you need it – and when you do need it, the Lifebridge B2T® can run for an incredible 2 hours without mains power.
The control unit has a conventional PC embedded in it as the “brains” of the system and the interface is a very slick touch sensitive flat panel and a rotary switch. I think the most surprising thing about Lifebridge B2T® to me was the user-friendly hardware engineering, and the intuitive Graphical User Interface that “gets everything just right.” You see the data you need when you need it, and you find the controls displayed where you want them when you want them. The rotary switch to control the pump speed is an astonishing concession to practicality and ergonomics over the “dazzling” menu driven technology which seems to be mandatory on every new consumer or medical device. When I want to turn down, or shut off a blood pump in a hurry, I do not want to be searching around on a glowing gas discharge display screen for a “virtual knob”- I want something I can instantly find, grab hold of, and twist!
Perhaps years-long exposure to US-made computer games has finally warped the GeNext German mind into sensible and understandable engineering. The Germans have always been able to manufacture beautifully crafted and engineered devices – the problem has been their often byzantine and madly complex implementations (beware of German plumbing!). The onboard PC records and stores all the typically desired case data and perfusion protocols. Patient specific data input is simple and straightforward, and there were USB ports on the machine I saw for data import/export.
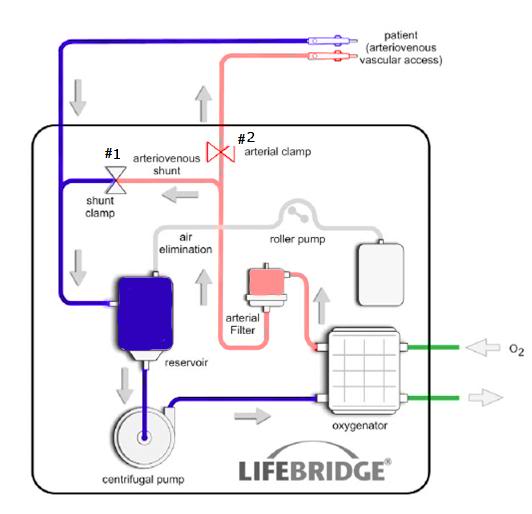 Figure 5: Schematic of the Lifebridge B2T® extracorporeal circuit. All of the components enclosed boxed area on the diagram are embed in the disposable, rugged and impact resistant disposable Patient Module.
Figure 5: Schematic of the Lifebridge B2T® extracorporeal circuit. All of the components enclosed boxed area on the diagram are embed in the disposable, rugged and impact resistant disposable Patient Module.
Applications
The system was designed primarily for Cardiac Catheterization lab or Emergency Department (ED) use by non-perfusionists to serve as a perfusion and oxygenation platform for patients who present in cardiogenic shock and who need the additional “time bridge” to get coronary revascularization via angioplasty, or coronary artery bypass grafting (CABG).8,9 It will also likely find ready application in treating profound hypothermia, where it is ideal for circulatory support and core rewarming. This is a significant issue on the Continent, where skiers and other winter outdoorsmen not infrequently experience exposure.
 Figure 6: At top, Members of the 59th Medical Wing Extrcorporeal Membrane Oxygenation Support Team undergoing hands-on ECMO training during an exercise on July 1, 2009, at Wilford Hall Medical Center, Lackland Air Force Base, Texas. The ECMO machine is a portable extended use cardiopulmonary bypass device that circulates and oxygenates the patient’s blood allowing time for acute lung injury or ARDS to resolve and the patient to recover. The lower picture is the current patient and ECMO hardware support cart mounted in a C-17 cargo plane. Anything that can reduce the weight and complexity of the ECMO system is invaluable under such conditions and ultra-compact and automated systems would allow for immediate application in forward, near battlefield positions.
Figure 6: At top, Members of the 59th Medical Wing Extrcorporeal Membrane Oxygenation Support Team undergoing hands-on ECMO training during an exercise on July 1, 2009, at Wilford Hall Medical Center, Lackland Air Force Base, Texas. The ECMO machine is a portable extended use cardiopulmonary bypass device that circulates and oxygenates the patient’s blood allowing time for acute lung injury or ARDS to resolve and the patient to recover. The lower picture is the current patient and ECMO hardware support cart mounted in a C-17 cargo plane. Anything that can reduce the weight and complexity of the ECMO system is invaluable under such conditions and ultra-compact and automated systems would allow for immediate application in forward, near battlefield positions.
I’ve been told that the US military has purchased Lifebridge B2T® units to evaluate in planned expanded deployment of extended (ECMO) support in cases of acute respiratory distress syndrome (ARDS) secondary to polytrauma, blunt force cardiac trauma and blast injury to the lungs (now “new” wartime pathologies as a result of body armor ‘softening the blow’ of what would have been otherwise lethal projectile impacts and much improve on site and in-field critical care medicine). The center for the military’s use of ECMO is the 59th Medical Wing at Wilford Hall at Lackland Air Force Base, TX. The ECMO capability at Wilford Hall dates back to the work of Air Force physician Gerald Klebanoff who invented the total body washout technique for treating stage IV hepatic coma in the early 1970s.10,11,12 The military commitment to this technology seems serious,13and in fact the Wilford Hall crew just had their first successful ECMO mission transporting a critically wounded soldier from Afghanistan back to Germany and then to the US for definitive treatment using a by C-17 cargo aircraft to carry the ECMO cart (Figure 6). This operation was planned, tasked, command and controlled by the 618th Air and Space Operations Center (AOC) at Scott Air Force Base, which is the principal US and NATO agency for worldwide military airlift, air refueling and aeromedical evacuation. The military is also beginning to use Novalung technology for acute stabilization and management of patients with respiratory failure secondary to battlefield trauma.14
The primary feature that will likely expand the use of CPB and ECMO into critical care and emergency medicine is the Lifebridge B2T®’s computerized “System for Prevention of Air Embolization (SPAE).” This system employs a 7-layer deep prevention protocol using an automatic “air management procedure” that is triggered by in blood macro and micro air detection employing an ultrasonic air bubble detector placed immediately after the arterial filter in the circuit.1 If bubbles are detected, the arterial line is closed within <300 msec by means of an arterial clamp (Figure 5, #1). By simultaneously opening, the arterio-venous (A-V) shunt line, which connects the arterial line (post filter) to the venous line (Figure 5, #2), blood containing micro- or macro-air is diverted to the venous reservoir without hazard to the patient. Once bubble removal is complete, the on-board computer closes the A-V shunt and unclamps the arterial line to once again begin perfusing the patient. The seven-stage SPAE operates as follows:
1) Air entering into the venous line, or re-circulating through the purge lines, rises to the top of the venous reservoir via the buoyancy effect, where the roller pump (Figure 5) actively removes it.
2) The venous reservoir employs a two compartment design separated by a120 micron membrane, or screen. One compartment handles the venous return, and the other serves as the sump from which blood is drawn for perfusion through the oxygenator and into the patient. The devising screen effectively excludes microbubble generated as a result of turbulence, negative pressure in the venous line, or air leaks around the venous cannula, from entering the arterial intake – in effect it is a pre-arterial filter microbubble filter – a very clever and long overdue safety feature.
3) Low venous blood level is detected by a sensor that immediately stops the pump. This system is fairly “smart” in that when I regularly interrupted venous return, the machine would shut down and re-start perfusion in a dynamic fashion in response to the blood level in the reservoir.
4) An inherent safety feature of centrifugal pumps is that they will not pump macro-air. The Lifebridge B2T® has cleverly built on this feature by mounting the centrifugal pump head with its tangential outlet pointing down, making it more difficult for any air entrained in the pump head to rise under the influence of gravity and enter the arterial line.
5) As is standard on all ECCS circuits, the oxygenator also serves as bubble trap and air can be eliminated by the automatic opening of the solenoid clamp on the oxygenator recirculation line, to divert flow to the venous reservoir where it can be vented or siphoned off as required.
6) Again, as is now universal practice, the arterial filter employs centrifugal air separation using spiral flow, and this air is continually eliminated from the circuit by a bleed line atop the filter, which is in turn controlled by the PC.
7) Whenever bubbles are detected behind the arterial filter, the “air management protocol” eliminates them, as previously described. All prevention measures are inherent in the configuration of the system except for 3) and 7) above, which are implemented in the control software by the PC.
The unit has no intrinsic heater/cooler, so an additional module to perform this function must be used if ECMO support is to go on for an extended period of time, or if rapid induction of therapeutic hypothermia is desired. The oxygenators used in this platform are all rated for 6 hour use, however, as any Third World perfusionist will tell you, in most cases they can be reliably run to 12 hours; and runs of 24 hours, or longer, are not uncommon. I would also note that if the era of profound or ultraprofound asanguineous hypothermic perfusion ever arrives in medicine, the useful life of microporous membrane oxygenators will be further extended, since the membrane retains its integrity much longer in the absence of both blood and normothermia. The Lifebridge B2T® system makes switching patient modules safe, rapid and easy, so in the event the oxygenator begins to weep transudate, clots too many fibers, or otherwise deteriorates in performance, switch-out is fast.
As with other Minimal Extracorporeal Circulation (mini-ECC) systems, the Lifebridge B2T® has superior blood handling characteristics. Due to reduced tubing volume there is correspondingly less prime needed, as well as a small reduction in the total surface area of foreign material that blood is exposed to, and thus less pro-inflammatory cytokine release.[2] The hemolysis characteristics of the system in clinical use are also, as expected, quite favorable, and there seems to have been no price paid for plumbing the system into such a constrained space.1
A testimony to the trust placed in the automated components of perfusion in the the Lifebridge B2T® is evidenced by the fact that much of the extracorporeal circuit is no longer visible to the operator. I found this very disconcerting, until I grew to trust the device’s “management” of perfusion.
The Future
I have long believed that an essential technology to a truly field-able ECC unit that can be pressed into service the instant vascular access is achieved in the field, is a pre-primed circuit incorporating a true membrane oxygenator. Such a circuit would be stored ‘wet,’ and be fully primed and ready for connection to the patient as soon as vascular access was established. With the advent of yet another German advance in extracorporeal technology, the development of the poly-4-methyl-1-pentene gas exchange membrane, now available clinically in the Jostra Rotaflow and QuadroxD true membrane hollow fiber oxygenators, this dream seems closer to a reality.15,16 However, the Lifebridge B2T® is forcing me to rethink this “requirement.” By compacting and cleverly arranging the circuit elements, priming and de-bubbling are made much simpler. There is no longer any need to beat on the arterial filter with a rubber reflex hammer, or to chase air from pillar to post in the circuit during priming. The machine is essentially self-priming. The only reason Medizintechnik didn’t make the device completely automated for priming is that it would have required the incorporation of a heavy, motorized tilt assembly to rock the circuit from the operational position to the priming position, and back again. This would clearly have been a poor engineering decision when that operation is so easily accomplished by human hands already present (and required) to operate the device. In practical terms, 5 minutes from start to patient ready, might as well be “instantaneous,” because vascular access will invariably take longer, far longer, in fact.
And that is just about the last barrier to the deployment of ECC and ECMO technology into first responder applications. There is no question that very rapid ECC would be highly effective in improving the outcome in sudden cardiac arrest if it could be applied on site, with effective CPR, such as that offered by the LUCAS device, being used only as a brief bridge in the event of failed first responder defibrillation.17,18,19 With further automation of ECC to minimize the requirement for highly developed quick reflexes in the operator, and further encoding in the machinery the complex algorithms for managing perfusion, the primary remaining barrier (aside from cost), is the inability to achieve rapid vascular access using minimally skilled personnel. This barrier seems insurmountable, but then so did the barriers to the creation of a device such as the Lifebridge B2T® as recently as a decade ago.
 Figure 7: An add-on cooling module using ammonium nitrate and water, similar to the technology used in “instant ice packs” or perflurochemical evaporative cooling as used in the RhinoChill, should easily be able to emergently reduce patient brain core temperature by ~ 3oC.
Figure 7: An add-on cooling module using ammonium nitrate and water, similar to the technology used in “instant ice packs” or perflurochemical evaporative cooling as used in the RhinoChill, should easily be able to emergently reduce patient brain core temperature by ~ 3oC.
One last point seems worth making and that is, regulatory considerations aside, it is easily possible to envision a very compact and disposable eutectic heat exchange module that could interface with the Lifebridge B2T® for the induction of Mild Therapeutic Hypothermia (MTH) in the field. Since only a 3oC drop in brain temperature is required acutely in MTH, the use of chilled prime solution (with another liter of chilled Ringer’s given at the start of bypass), a pre-cooled Patient Module, and the addition of a eutectic heat absorbing source, such as ammonium nitrate activated by water, or an evaporative PFC azeotrope such as is used in the RhinoChill,20,21 it should be possible to induce MTH in the field, or en route to the hospital without recourse to ice, or heavy refrigeration systems.
References
1) Mehlhorn U, Brieske M, Fischer UM, Ferrari M, Brass P, Fischer JH, Zerkowski HR. LIFEBRIDGE: a portable, modular, rapidly available “plug-and-play” mechanical circulatory support system. Ann Thorac Surg. 2005 Nov;80(5):1887-92. PubMed PMID: 16242474.
2) Krane M, Mazzitelli D, Schreiber U, Garzia AM, Braun S, Voss B, Badiu CC, Brockmann G, Lange R, Bauernschmitt R. LIFEBRIDGE B2T–a new portable cardiopulmonary bypass system. ASAIO J. 2010 Jan-Feb;56(1):52-6. PubMed PMID: 20051839.
3) Maunz O, Horisberger J, von Segesser L. Bridge to life: the Lifebridge B2T extracorporeal life support system in an in vitro trial. Perfusion. 2008 Sep;23(5):279-82. PubMed PMID: 19346266.
4) Camboni D, Philipp A, Arlt M, Pfeiffer M, Hilker M, Schmid C. First experience with a paracorporeal artificial lung in humans. ASAIO J. 2009 May-Jun;55(3):304-6. PubMed PMID: 19282751.
5) Kopp R, Bensberg R, Henzler D, Niewels A, Randerath S, Rossaint R, Kuhlen R. Hemocompatibility of a miniaturized extracorporeal membrane oxygenation and a pumpless interventional lung assist in experimental lung injury. Artif Organs. 2010 Jan;34(1):13-21. Epub 2009 Oct 11. PubMed PMID: 19821813.
6) Ricci D, Boffini M, Del Sorbo L, El Qarra S, Comoglio C, Ribezzo M, Bonato R, Ranieri VM, Rinaldi M. The use of CO2 removal devices in patients awaiting lung transplantation: an initial experience. Transplant Proc. 2010 May;42(4):1255-8. PubMed PMID: 20534274.
7) Fernández P, Muñoz P, Fischer D, Méndez F, Florenzano M, Valdés S, Parada MT, Fica M, Rodríguez P, Díaz R, Rufs J. [Bridge to lung transplantation with a novel pumpless lung assist device. Report of one case]. Rev Med Chil. 2009 Oct;137(10):1363-6. Epub . Spanish. PubMed PMID: 20011945.
8) von Segesser LK, Kalejs M, Ferrari E, Bommeli S, Maunz O, Horisberger J, Tozzi P. Superior flow for bridge to life with self-expanding venous cannulas. Eur J Cardiothorac Surg. 2009 Oct;36(4):665-9. Epub 2009 Jul 16. PubMed PMID: 19615916.
9) Jung C, Schlosser M, Figulla HR, Ferrari M. Providing macro- and microcirculatory support with the Lifebridge System during high-risk PCI in cardiogenic shock. Heart Lung Circ. 2009 Aug;18(4):296-8. Epub 2008 Aug 31. PubMed PMID: 18762457.
10) Klebanoff G, Armstrong RG, Cline RE, Powell JR, Bedingfield JR. Resuscitation of a patient in State IV hepatic coma using total body washout. J Surg Res. 1972 Oct;13(4):159-65. PubMed PMID: 5078628.
11) Cline RE, Klebanoff G, Armstrong RG, Stanford W. Extracorporal circulation in hypothermia as used for total-body washout in stage IV hepatic coma. Ann Thorac Surg. 1973 Jul;16(1):44-51. PubMed PMID: 4721190.
12) Klebanoff G, Langdon D, Wilen S, Tobias H. Total-body washout in hepatic coma. N Engl J Med. 1973 Oct 11;289(15):807. PubMed PMID: 4728761.
13) Midla GS. Extracorporeal circulatory systems and their role in military medicine: a clinical review. Mil Med. 2007 May;172(5):523-6. Review. PubMed PMID: 17521103.
14) Bein T, Osborn E, Hofmann HS, Zimmermann M, Philipp A, Schlitt HJ, Graf BM. Successful treatment of a severely injured soldier from Afghanistan with pumpless extracorporeal lung assist and neurally adjusted ventilatory support. Int J Emerg Med. 2010 Jul 13;3(3):177-9. PubMed PMID: 21031042; PubMed Central PMCID: PMC2926866.
15) Mongero LB, Brodie D, Cunningham J, Ventetuolo C, Kim H, Sylvan E, Bacchetta MD. Extracorporeal membrane oxygenation for diffuse alveolar hemorrhage and severe hypoxemic respiratory failure from silicone embolism. Perfusion. 2010 Jul;25(4):249-52; discussion 253-4. Epub 2010 Jun 21. PubMed PMID: 20566586.
16) Horton S, Thuys C, Bennett M, Augustin S, Rosenberg M, Brizard C. Experience with the Jostra Rotaflow and QuadroxD oxygenator for ECMO. Perfusion. 2004 Jan;19(1):17-23. PubMed PMID: 15072251.
17) Greisen J, Golbaekdal KI, Mathiassen ON, Ravn HB. [Prolonged mechanical cardiopulmonary resuscitation]. Ugeskr Laeger. 2010 Nov 15;172(46):3191-2. Danish. PubMed PMID: 21073835.
18) Larsen AI, Hjørnevik A, Bonarjee V, Barvik S, Melberg T, Nilsen DW. Coronary blood flow and perfusion pressure during coronary angiography in patients with ongoing mechanical chest compression: a report on 6 cases. Resuscitation. 2010 Apr;81(4):493-7. PubMed PMID: 20227005.
19) Wagner H, Terkelsen CJ, Friberg H, Harnek J, Kern K, Lassen JF, Olivecrona GK.Cardiac arrest in the catheterisation laboratory: a 5-year experience of using mechanical chest compressions to facilitate PCI during prolonged resuscitation efforts. Resuscitation. 2010 Apr;81(4):383-7. Epub 2009 Dec 14. PubMed PMID: 20007005.
20) : Boller M, Lampe JW, Katz JM, Barbut D, Becker LB. Feasibility of intra-arrest
hypothermia induction: A novel nasopharyngeal approach achieves preferential brain cooling. Resuscitation. 2010 Aug;81(8):1025-30. Epub 2010 Jun 9. PubMed PMID: 20538402.
21) Busch HJ, Eichwede F, Födisch M, Taccone FS, Wöbker G, Schwab T, Hopf HB, Tonner P, Hachimi-Idrissi S, Martens P, Fritz H, Bode Ch, Vincent JL, Inderbitzen B, Barbut D, Sterz F, Janata A. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation. 2010 Aug;81(8):943-9. Epub 2010 Jun 2. PubMed PMID:20627524.
[1] Bullith Batteries, Ismaning, Germany
[2] The primary source of blood exposure to non-native surfaces remains the oxygenator-heat exchanger and the arterial filter, both of which have enormous surface areas which are necessary for them to perform their functions.

Interesting.
Is this device legal in the U.S.? If not, what regulatory hurtles must be overcome to make it available for use in the U.S.?
That’s a weird story, and I don’t have time to tell it all here. The hand-held CardioPump is still illegal to be sold in the US. It used to be quite hard to get them, and I had the first ones sent into the US via the efforts of someone in the UK who was kind enough to ship them as “plastics stress testing devices.” Ambu in the US actually kept their devices under lock and key in New York (no kidding). The reason they are illegal is that they are an FDA unapproved medical device. The reason they are unapproved is that they failed two clinical trials conducted in the US:
Cohen TJ, Goldner BG, Maccaro PC, Ardito AP, Trazzera S, Cohen MB, Dibs SR. A comparison of active compression-decompression cardiopulmonary resuscitation with standard cardiopulmonary resuscitation for cardiac arrests occurring in the hospital. N Engl J Med. 1993 Dec 23;329(26):1918-21. PubMed PMID: 8018138.
Ellinger K, Luiz T, Denz C, van Ackern K. [Randomized use of an active compression-decompression technique within the scope of preclinical
resuscitation]. Anasthesiol Intensivmed Notfallmed Schmerzther. 1994 Dec;29(8):492-500. German. PubMed PMID: 7841276.
The Cohen study is available as a free full text here: http://www.nejm.org/doi/pdf/10.1056/NEJM199312233292603
If you delve deeply into CPR research, you will find that almost everything fails – including interventions or devices that unequivocally improve perfusion and oxygenation. In fact, most of these interventions, such as CPR machines, show a worse outcome! So how is this possible if blood flow is better using the devices? Several answers have been proposed, but here are the most likely ones:
1) The application of any modality that requires a device almost invariable translates into either more time doing CPR, or interruptions in CPR to apply the device. You can’t put most mechanical CPR machines on the patient without, however briefly, interrupting CPR. That is a no, no! Even a 5 second interruption in CPR markedly negatively impacts outcome. This is one of the reasons that Gordon Ewy’s “new” compressions only “cardiopulmonary cerebral resuscitation CPR” is working – and forcing the American Heart Association into a very uncomfortable position of retreat.
Every time you interrupt CPR to give a breath, flow stops, and that is very injurious. What’s more, even if you give the breath while continuing CPR you are transiently raising the pressure inside the chest. CPR does not move blood primarily by squeezing the heart “between the breastbone and the backbone” as the lay literature says. Rather, it works using other mechanisms, primary something called the “thoracic pump” and possibly something called the “lung pump.” These mechanisms depend upon a negative pressure being created within the chest which in turns pulls or “sucks” blood into the great vessels of the thorax which is subsequently expelled during the down-stroke part of the CPR duty cycle. When you give a breath in CPR you are giving a positive pressure breath. That is unphysiologic, because if you think about it, every time you inhale normally, you are creating a negative pressure (relative vacuum) inside the chest by pulling your diaphragm downwards. Each positive pressure breath prevents or actively expels blood from the chest – effectively cutting into cardiac output.
The vast majority of people who are going to survive cardiac arrest do so as a result of early defibrillation, not CPR. Mostly, CPR doesn’t work, and the proof of this is the proliferation of all these expensive automatic external defibrillators (AEDs) in airports, shopping malls, and the like. And that brings us to reason #2:
2) Patients who get “devices” to assist with CPR are usually those who have failed the first round of AED. That is a very, very bad prognosis, because every minute you spend absent spontaneous circulation is pretty much ischemic time, and by the time the new device is brought into the picture, your 4-6 minute of cerebral salvage-ability has likely clocked out.
3) There is a shallow learning curve to using all of these devices. I know, I’ve used them. It takes lots of continued practice to apply even the the easiest to apply of these devices without losing time to no flow. In fact, you wouldn’t believe how much effort is required – and it is ongoing, because if you don’t keep drilling, you lose speed. Paramedics HATE CPR machines and don’t much like CPR adjuncts, either. It is hard to describe the degree of their contempt. It seems to be largely an artifact of their belief that they are better than any machine. This doesn’t make it easy to get good compliance during clinical trials. And CPR isn’t that commonplace – who is going to spend time drilling with a noisy, clunky machine 2x a week…?
4) The CardioPump and other active decompression CPR devices really only work well if you close the airway during upstroke! Since you are trying to create a relative vacuum in the chest during upstroke by pulling on the chest wall with a suction cup, it hardly makes sense to allow air to rush into the lungs 100 time/min, and defeat this process. It also results in over-ventilation, which is very bad in CPR. So, a resuscitation researcher named Keith Lurie invented the airway impedance valve, or as it now called, an “impedance threshold device (ITD)”. This device closes the airway during upstroke and allows for breaths to be given using positive pressure ventilation, as needed. A couple of the papers below are full text and will explain the mechanics of the ITD:
Plaisance P, Soleil C, Lurie KG, Vicaut E, Ducros L, Payen D. Use of an inspiratory impedance threshold device on a face mask and endotracheal tube to reduce intrathoracic pressures during the decompression phase of active compression-decompression cardiopulmonary resuscitation. Crit Care Med. 2005 May;33(5):990-4. PubMed PMID: 15891326.
Lurie KG, Barnes TA, Zielinski TM, McKnite SH. Evaluation of a prototypic inspiratory impedance threshold valve designed to enhance the efficiency of cardiopulmonary resuscitation. Respir Care. 2003 Jan;48(1):52-7. PubMed PMID:12556262.
http://www.rcjournal.com/contents/01.03/01.03.0052.pdf
Lurie K, Zielinski T, McKnite S, Sukhum P. Improving the efficiency of cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Crit Care Med. 2000 Nov;28(11 Suppl):N207-9. PubMed PMID: 11098948.
Lurie K, Voelckel W, Plaisance P, Zielinski T, McKnite S, Kor D, Sugiyama A, Sukhum P. Use of an inspiratory impedance threshold valve during cardiopulmonary resuscitation: a progress report. Resuscitation. 2000 May;44(3):219-30. Review. PubMed PMID: 10825624.
Plaisance P, Lurie KG, Payen D. Inspiratory impedance during active compression-decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. Circulation. 2000 Mar 7;101(9):989-94. PubMed PMID: 10704165. http://circ.ahajournals.org/cgi/reprint/101/9/989
This work resulted in a clinically available FDA approved device called the ResQPod, and there is a very nice series of animated teaching modules showing how it works here:
http://www.advancedcirculatory.com/resqpod/product_overview.htm
Addition info can be had here:
http://www.mypatrioteducation.com/classes/resqpod/ResQPOD_FAQs.pdf
But, to return to the CardioPump. Since the device failed its clinical trials it not allowed, however, a mechanical CPR device called the LUCAS, which uses the CardioPump suction cup is FDA approved and can be purchased in the US. You can watch an instructional video for the LUCAS here:
http://www.youtube.com/watch?v=3M96sFP-0Xc&NR=1
The LUCAS + ITD is the most effective CPR available, bar none, and I am hearing first-hand reports of patients being conscious on LUCAS support absent a spontaneous heartbeat more and more, almost by the month, from physicians using it in Europe. It is rapidly becoming the standard of care in the Netherlands. LUCAS recently came out with an electrically powered unit (an artifact of LiPO battery technology) which eliminates the need for a bulky and difficult to transport oxygen tanks. — Mike Darwin
In figure 6 you have the bottom picture labeled as the ECMO hardware mounted in a C17 cargo plane. It is actually a picture of the second generation of arkansas children’s mobile ecmo stretcher in a lear jet. Just wanted to correct that.