

By Mike Darwin
The problem of a cryonicist experiencing sudden cardiac arrest (SCA) unattended is hardly theoretical. This has occurred a number of times already, with some patients going upwards of a week before being discovered. Because SCA is only reversible within the very narrow time frame of 4-6 minutes after circulation stops, there has been little incentive to detect it, and instantly relay a call for help, outside of hospital, that is. By the time help arrived, even if it was just 5 or 10 minutes away, it would be too late. This sad state of affairs has been a source of enormous frustration for cryonicists since at least 1981 – that’s when I saw the first prototype for a wearable cardiac arrest monitoring alarm system. It had been constructed by Reg Thatcher, who was then associated with Jerry Leaf’s Cryovita Labs, in Fullerrton, CA; and it worked. It was a marvel of engineering for its time, but no one wanted to use it. It was a bit cumbersome, the wet gel electrodes caused skin irritation, and its range was very limited.
Since Reg’s efforts, a number of people in cryonics have tried to engineer a solution to this problem, most notably Ben Best and Eugen Leitl. There is no question that the technological base exists. In fact, it has existed since at least 1981, and from the standpoint of feasibly, miniaturizing such a system to the degree that it would be tolerable for most users to wear and be compliant in its use; it has probably been possible since at least 2000. This technology has been developed not because of life or death issues, but because of sports. Shown in Figure, 1, below are three implementations of sports heart-rate monitors with high and low alarm features.
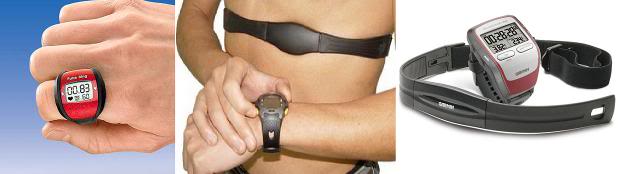
Figure 1: At left is finger-worn sports-type digital pulse monitor which use pulse oximetery technology to detect hear beat. Center,above, is an ECG-type sports watch; the chest belt contains the ECG electrodes and transmitter which sends the heat rate count to the wrist-worn watch. At right is the Garmin Forerunner 305 GPS Heart Rate Monitor which also has full GPS capability!
The one the right, the Garmin Forerunner 305 GPS Heart Rate Monitor, is not merely a continuous heart rate monitor; it is a fully capable GPS navigator. It also calculates calorie consumption based on the user’s weight, distance traveled and hills climbed, records lap history by day and week, stores up to 200 workouts in its memory and, in addition to heart rate alarms, it also has something called a “pace alarm,” which sounds if the user is running either faster or slower than their programmed pace! Oh yes, I almost forgot, it has time/distance alarm alerts when you’ve reached a desired time or distance and it sells for a mere ~ $200 US. Other manufacturers sell similar monitors with Bluetooth capability, thus allowing the watch output to be linked to mobile phones or other Bluetooth enabled devices. For example the the Nokia Symbian S60 Smartphone shown in Figure 2, below, can even be bundled with a Bluetooth linked Polar heart rate monitor and alarm.[1]
 Figure 2: The Nokia Symbian S60 Smartphone with companion Bluetooth linked Polar heart rate monitor: yours for ~ $700, US.
Figure 2: The Nokia Symbian S60 Smartphone with companion Bluetooth linked Polar heart rate monitor: yours for ~ $700, US.
So what’s the problem? Why isn’t the issue of an alarm system to detect SCA for cryonicists “a done deal?” The answer is that because of US Food and Drug Administration (FDA) regulations, the devices may not use the alarm feature for anything beyond sports training purposes – and there is no sport training reason to have the phone dial a preprogrammed number in the event of cardiac arrest. The downside to this kind elegant, compact and integrated technology as seen in Figures 1 and 2 is that it is increasingly beginning to look and act like biological systems do. It isn’t possible to peel the top of your dog’s head off and rewire him to bark only at certain times, or to stop him from scratching at the door when you want to be alone. Try to do that, and all you’ll find inside is a bunch of amorphous looking goo which you can make no sense out of. And of course, your dog will either be dead, or very, very irritated with you.
The insides of the Nokia phone or Garmin Forerunner now look a lot like the insides of a living system, and it is no longer possible to easily hack, reroute, rewire or otherwise modify their function. The potentially positive side to the Nokia Symbian is that it is a Smartphone, and therefore (again in theory) it may be possible for a skilled Smartphone applications programmer to create an “app” that would autodial the phone to a predetermined series of numbers in the event of a cardiac arrest event. Presumably the speed dial button on the phone could be used to facilitate “manual” panic alarm calls. If such an app were to prove possible, then the only uncovered contingency would be a fall that leaves the person alive, but unable to call for help. This is, unfortunately, a common scenario in stroke.
Vitalsens Vital Signs Monitor
Very recently, cardiac arrest-fall-panic alarms for medical applications that meet FDA or CE (European Union) standards have been developed. So now, 30+ plus years after cryonicists made their first attempt at it, a new range of products is entering the marketplace that will more or less do the job – and in one case do considerably more.
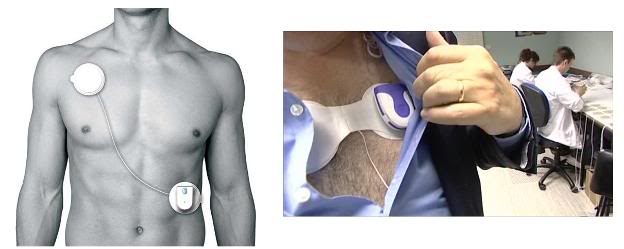
Figure 3: The Vitalsens remote cardiac monitor and electrode array by Vitalsens Technologies of Dublin, Ireland.
The first of these is the Vitalsens ‘plug and play’ Vitalsens vital signs monitor.[1] This is a device I’ve been waiting for since Intelesens released its V-Patch Cardiac Event Monitor, the V-Patch Medical Systems (VPMS) in 2008. The Vitalsens contains transducers for ECG, heart rate, and skin temperature which are integrated with a tiny transmitter. The unit sticks to the skin with a minimally irritating disposable adhesive pad and is worn until the user removes it. The ECG electrodes and skin temperature sensor are disposable. The transmitter component of the Vitalsens monitor also contains a motion sensing device employing a 3-axis accelerometer – this allows the device to detect “man down” situations, such as a sudden fall, even when there is no cardiac arrest or arrhythmia. Most importantly, the Vitalsens is an ”open-source” OEM[2] device designed to transmit data via Bluetooth to a PC, or any Bluetooth compatible device; and Intellisens provides it along with PC interface software. This allows the Vitalsens to be interfaced to any existing or new monitoring product. It can thus be rapidly added to or integrated into existing medical devices or alarm/monitoring systems.
What this means in theory is that it should be possible for any cryonicists with real engineering savvy out there to adapt another device, such as a smartphone, to respond to a signal from the Vitalsens. While still no easy task, it is unarguably much better than trying to build such a system de novo, or to rewire or reprogram a Nokia Symbian S60 – or your dog. More information on the Intelisens system is available at : http://intelesens.com/index.html
The NUVANT Mobile Cardiac Telemetry System
Next up is the The NUVANT Mobile Cardiac Telemetry System from Corventis, Inc., in San Jose, CA. Corventis got 510(k) FDA approval for this system early last year, and it is now up and running. The NUVANT system uses a disposable sensor array and transmitter which they call the PiiX (Figure 4, below); “an unobtrusive, leadless and water-resistant device “designed to support patient compliance.” The PiiX transmits its data to a compact relay transmitter, the zLink, that can be worn on the belt, or otherwise be secured to the user’s person. The word ‘patient’ should probably be used here because this is an FDA approved prescription device.
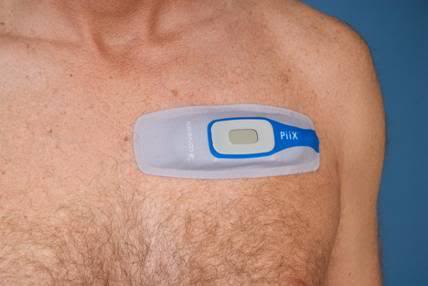
Figure 4: The Corventis NUVANT PiiX is an unobtrusive, leadless and water-resistant ECG sensor and transmitter, which is also disposable. It links to a small, belt-worn re-transmitter, the zLink, which in turn communicates using mobile phone technology with Corventis’ central monitoring station.
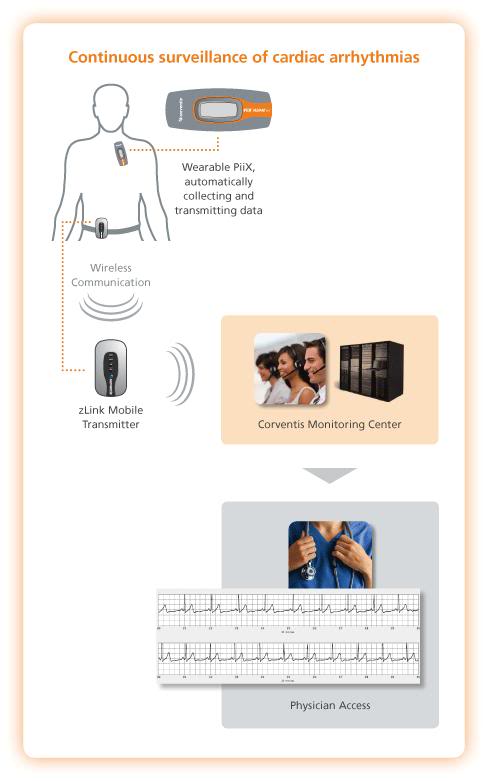
Figure 6: The NUVANT monitoring system works by activating a belt-worn unit which communicates the arrhythmia to a central monitoring station, where it is interpreted and acted upon as deemed necessary by ECG technicians.
The NUVANT technological platform was developed primarily as a competing technology to Holter monitoring, with the added advantage of 24 hour centralized monitoring of the ECG. When the device detects an arrhythmia, it activates the belt-worn transmitter and notifies ECG monitoring technicians at the central monitoring station, who can then review the arrhythmia and advise the patient directly, or the patient’s cardiologist, as appropriate. If the patient experiences symptoms, he can activate PiiX to record and automatically transmit the ECG, via the zLink transmitter device, to the Corventis Monitoring Center.
Corventis offers three levels of service, as shown in Figure 7, below. Costs vary considerably for monitoring service, depending upon the level of surveillance selected. When the device detects cardiac arrest, or a rapidly fatal arrhythmia, such as ventricular tachycardia in a patient, it captures it and sends the ECG virtual “strip” to the Corventis central monitoring facility for action or referral to the patient’s cardiologist.
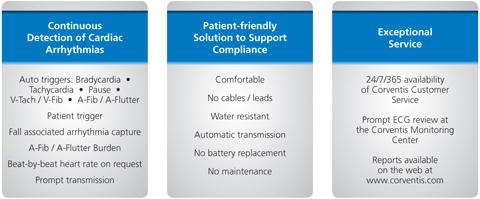
Figure 7: Varying service levels available for NUVANT monitoring system.
The service is geared primarily for detection of arrhythmias for diagnostic purposes, although a stated use of the device is to monitor for acute therapeutic interventions, presumably including summoning Emergency Medical Personnel (EMS).
The Zoll LifeVest Wearable Automatic Defibrillator
The bulk of my research activity has not been in cryobiology, but rather in the area of cerebral-ischemia reperfusion injury[2] (lack of blood flow to the brain and the injury that results from this, and as a consequence of restoring blood circulation) and the “post-resuscitation syndrome.”[3] The brain is acutely and atypically sensitive to lack of blood flow. The outer limit of recovery from cardiac arrest absent neurological deficit is 6 minutes, and the vast majority of patients cannot be recovered from cardiac arrest in any condition, if the duration is as little as 10 minutes.[4] Independent of the brain injury associated with ischemia, there is also a ~ 10% decrease in survival for each minute that cardiac arrests persists absent restoration of spontaneous circulation (ROSC). That number should put into perspective why the immediate detection of cardiac arrest is such a low commercial and medical priority – the window of time to act is just too small!
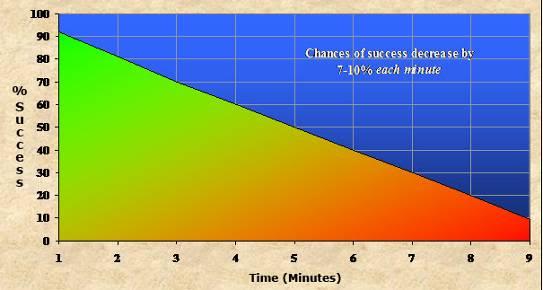 Figure 8: The graph above shows the smoothed curve for chances of survival (with and without neurological deficit) as a function of time in cardiac arrest until there is effective restoration of spontaneous circulation (ROSC). Beyond 5 minutes of cardiac arrest the neurological outcome becomes progressively worse, as indicated by shading into red on the graph. The first incidents of impaired neurocognitive function following cardiac arrest occur starting ~ 2 minutes after the start of aystole.[4-8]
Figure 8: The graph above shows the smoothed curve for chances of survival (with and without neurological deficit) as a function of time in cardiac arrest until there is effective restoration of spontaneous circulation (ROSC). Beyond 5 minutes of cardiac arrest the neurological outcome becomes progressively worse, as indicated by shading into red on the graph. The first incidents of impaired neurocognitive function following cardiac arrest occur starting ~ 2 minutes after the start of aystole.[4-8]
Each year ~300,000 people in the US will experience SCA[9] and ~70% of them will not be within reach of an Automatic External Defibrillator.[10] Of the 30% who do experience SCA within “potential” reach of an AED, the majority of these patients will not be salvageable due to delays involved in determining the patient’s condition (i.e., the bystander determining if the victim in fact have no heartbeat), locating an AED, applying it, and waiting for it to diagnose the arrhythmia and administer the anti-arrhythmic shock.[11] Once again, the logistically imposed time delay in the context of the decay curve in recovered viability, as shown in Figure 8 above, means that only a minority of patients who need it will benefit from rapid, let alone immediate defibrillation.
For at least a decade now, implantable cardiac defibrillators (ICDs) have been available.[12, 13] These units place the entire defibrillator assembly under the skin of the chest and within the patient’s thorax. They work well, but the procedure to implant them is both invasive and costly, and the batteries have a limited life, requiring re-operation every few years to replace them.[14] Because the devices and the surgical and medical management associated with their use are so costly, insurance carriers will typically only pay for ICDs in patients who have both extremely high and documented ongoing risks of sudden cardiac arrest.
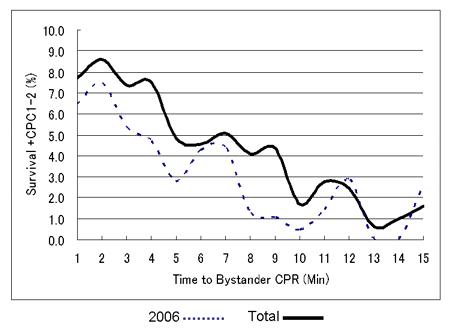 Figure 9: This is very recent data (2006-07) from All-Japan Utstein Registry of the Fire and Disaster Management Agency. They selected a subpopulation of SCA their patients with out-of-hospital cardiac arrest due to ventricular fibrillation (VF), where there was witness present who was over 18 years of age and who immediately started CPR. The study ran from January 1, 2006 to December 31 2008 and the outcomes measured were survival and good neurological outcome (CPC 1 or 2). There were 24479 adults in the analysis with a mean time to bystander CPR of 1 minute. Adjusted odds ratio of the average effect per a minute was 0.23 with 95% Confidence Intervals (CI) 0.17 to 0.31.The introduction of high impulse no-pause for ventilation CPR was associated with a significant improvement in survival despite the fact that it is still not in widespread use. As can be seen, even with essentially immediate initiation of bystander CPR, survival from cardiac arrest is not greatly improved over that achieved with defibrillation, as seen in Figure 8, above. Abstract 260: The Effect of Time to Bystander Cardiopulmonary Resuscitation on Survival From Out-of-hospital Cardiac Arrest From All-Japan Utstein Registry Data: A Validation of 3-Phase Sensitive Model. Yonemoto, N, Yokoyama, H, Nagao,K, Kimura,T, Hiroshi,N. Circulation. 2010;122:A260
Figure 9: This is very recent data (2006-07) from All-Japan Utstein Registry of the Fire and Disaster Management Agency. They selected a subpopulation of SCA their patients with out-of-hospital cardiac arrest due to ventricular fibrillation (VF), where there was witness present who was over 18 years of age and who immediately started CPR. The study ran from January 1, 2006 to December 31 2008 and the outcomes measured were survival and good neurological outcome (CPC 1 or 2). There were 24479 adults in the analysis with a mean time to bystander CPR of 1 minute. Adjusted odds ratio of the average effect per a minute was 0.23 with 95% Confidence Intervals (CI) 0.17 to 0.31.The introduction of high impulse no-pause for ventilation CPR was associated with a significant improvement in survival despite the fact that it is still not in widespread use. As can be seen, even with essentially immediate initiation of bystander CPR, survival from cardiac arrest is not greatly improved over that achieved with defibrillation, as seen in Figure 8, above. Abstract 260: The Effect of Time to Bystander Cardiopulmonary Resuscitation on Survival From Out-of-hospital Cardiac Arrest From All-Japan Utstein Registry Data: A Validation of 3-Phase Sensitive Model. Yonemoto, N, Yokoyama, H, Nagao,K, Kimura,T, Hiroshi,N. Circulation. 2010;122:A260
This leaves a lot of people with known risks with no protection against SCA other than bystander CPR and the Emergency Medical System (EMS). Representative survival following cardiac arrest under excellent conditions of bystander and EMS response is shown in Figure 9, above and it is not greatly different than when the return of spontaneous circulation is achieved by the use of defibrillation, even absent CPR (Figure 8).[5, 7] The focus of my research towards solving this problem has been to find better methods of CPR as well as drugs that will allow for cerebral recue following periods of brain ischemia in the range of 6-30 minutes. My reason for focusing on these strategies is that because cryopreservation procedures cannot be started on humans until after pronouncement of medico-legal death, all cryonics patients will necessarily experience some normothermic ischemia. Furthermore, since it is also not legally permissible to restore spontaneous circulation to cryonics patients, they will necessarily be reliant upon some form for closed-chest circulatory support. at least until such time as extracorporeal support can be initiated.
Medical patients are not constrained to suffer “irreversible cessation” of spontaneous circulation following an incident of SCA and they can thus legally, if not practically, be immediately defibrillated. However, as has just been pointed out, only those at the highest risk qualify for ICD placement. On average, the long-term risk of sudden death after myocardial infarction (MI) (once a person has been medically stabilized for ~ 30-60 days), is between 1 – 2% per year.[15] During the first 30 days after an MI the risk is in the range of 25 to 50% and for a subpopulation of MI patients, the risk remains high. The patients with the highest risk are those who have already had (and survived) one episode of cardiac arrest; they face a ~ 20% yearly chance of another cardiac arrest. Patients with a large MI, ones in whom there has been destruction of a large area of heart muscle, also face a seriously elevated risk of SCA. A good marker for the amount of the myocardium destroyed after an MI is the ejection fraction.[16] Patients with ejection fractions above 40% have about the same risk as other post-MI patients, a ~ 1 – 2% incidence of SCA per year.[16] However, the risk of sudden death increases with lower ejection fractions, and becomes substantially higher at values of 30% or below. For this reason, anyone who has had a heart attack should have their ejection fractions measured – and should know what their ejection fraction is. Other patients who will be at greatly elevated risk, are those with infarcts in certain areas of the heart, those with co-morbidities such as diabetes, advanced age with atherosclerosis, and those with cancers and a prior history of MI.
To meet the need of these patients, particularly those who are in the high risk window period following an MI, the LifeVest wearable cardiac defibrillator (WCD) was developed by Zoll Medical (the same company that now manufactures the AutoPulse vest-CPR machine[3]).The LifeVest is the first wearable defibrillator, and in contrast to an ICD, the LifeVest is worn outside the body, rather than being implanted in the chest, as shown in Figure 10, below.[17-20]
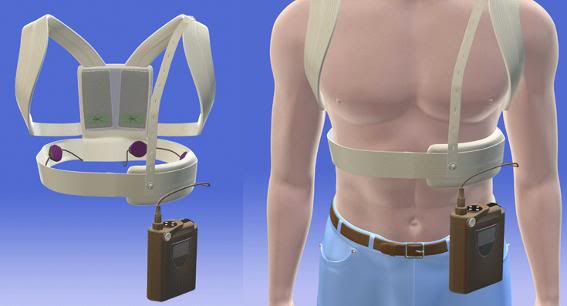
Figure 10: The Zoll LifeVest wearable cardiac defibrillator consist of a halter-worn set of monitoring and defibrillation electrodes and a shoulder strap or belt-worn monitor, electronics package and battery pack.
The LifeVest continuously monitors the patient’s ECG with novel, dry, non-adhesive, non-irritating sensing electrodes to detect potentially lethal cardiac arrhythmias. If a life-threatening rhythm is detected, the device alerts the patient prior to delivering a counter-shock, thus allowing patients who are conscious to delay the treatment shock, or to prepare themselves physically and psychologically; for instance, to sit down if standing, or to pull over to the side of the road, if driving. If the patient becomes unconscious, the LifeVest defibrillation electrodes secrete an electroconductive gel and deliver an electrical shock to restore normal rhythm. Approximately 35,000 patients have used the LifeVest so far, with a 98% cardioversion rate to normal rhythm on the first shock.[21, 22] The overall survival rate is 92%, with patients either remaining at home following the shock, or admitted conscious to the Emergency Department (ED).[18, 23]
Since its release in 2002, when the device was manufactured by its original developer LifeCor (subsequently acquired by Zoll Medical) it has gone through 4 generations of development, with each generation resulting in more compact and lighter weight units.
The LifeVest is covered by most health plans in the United States, including commercial, state, and federal plans as Durable Medical Equipment (DME) for those patients at high risk of cardiac arrest, including:
- Primary prevention [ Ejection fraction (EF) ≤35% and Myocardial Infarction (MI), Non Ischemic Cardiomyopathy (NICM), or other Dilated Cardiomyopathy (DCM)] including:
- After recent MI (Coverage during the 40-day ICD waiting period)
- Before and immediately after CABG or PTCA (Coverage during the 90-day ICD waiting period)
- Listed for cardiac transplant
- Recently diagnosed NICM (Coverage during the three-to-nine month ICD waiting period)
- New York Heart Association (NYHA) Class IV heart failure
- Terminal disease with life expectancy of less than one year
- ICD indications when patient condition delays or prohibits ICD implantation
- ICD explantation (patients who have had removal of an ICD but who are stillest risk of SCA)
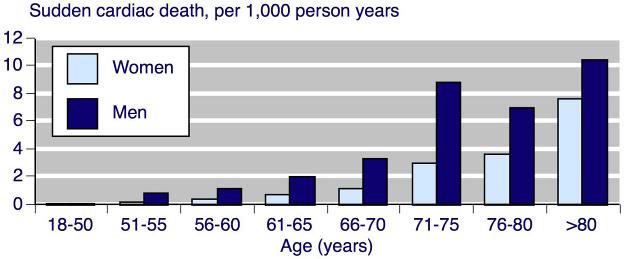
Figure 11: The risk of sudden cardiac death (not successfully resuscitated) per 1,000-person years for men and women by age.[24]
Can Cryonicists Benefit from a WCD?
While the indications for use of a WCD like the LifeVest are clear in cardiac disease, what, if any, are the indications in the cryonics setting? Figures 11-13 should help to clarify that somewhat. As can be calculated from Figure 11, the cause of death in 4 of every 5 people older than 65, is heart disease. Some of these deaths will be due to end-stage congestive heart failure, but many will result from SCA. These data would suggest that male cryonicists, in particular those with a family history of cardiac disease, are people who should at least consider the use of WCD after age 70. Mortality from SCA is so high by >75 years that very serious consideration should be given to use of a WCD in both men and women.[15, 25]
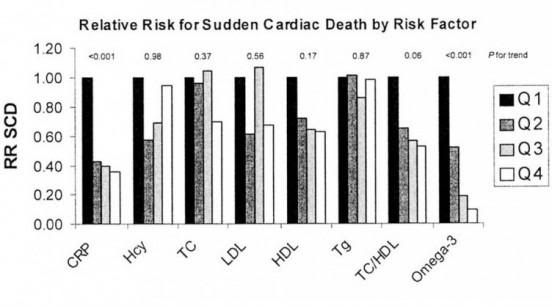 Figure 12: The multivariable-adjusted relative risk for sudden cardiac death by quartile of the Omega-3 Index compared with other, more traditional circulating heart risk factors. The quartiles at presumed highest risk (black bars) are set at a relative risk of 1.0. Each subsequent lighter bar represents the risk at each decreasing (or, for HDL and omega-3 index, increasing) quartile. CRP, C-reactive protein; Hcy, homocysteine; TC, total cholesterol; Tg, triglycerides. Source: Physician’s Health Study. These risks factors, combined with others, may allow younger cryonicists to determine their risk of SCA, and thus decide whether to make use of WCD.
Figure 12: The multivariable-adjusted relative risk for sudden cardiac death by quartile of the Omega-3 Index compared with other, more traditional circulating heart risk factors. The quartiles at presumed highest risk (black bars) are set at a relative risk of 1.0. Each subsequent lighter bar represents the risk at each decreasing (or, for HDL and omega-3 index, increasing) quartile. CRP, C-reactive protein; Hcy, homocysteine; TC, total cholesterol; Tg, triglycerides. Source: Physician’s Health Study. These risks factors, combined with others, may allow younger cryonicists to determine their risk of SCA, and thus decide whether to make use of WCD.
Cryonicists with diabetes who have suffered a MI have a 40% risk of experiencing lethal SCA in the following year (Figure 13, below).[26] That risk declines some with survival to the end of the second year, but still remains above 30% for the rest of the patient’s life! This subpopulation of cryonicists might also want to consider use of a WCD, or if medically indicated, an ICD. It is, of course, possible to greatly refine the risks of SCA by doing testing, such as cardiac “treadmill” testing wherein the ECG is monitored (preferably in conjunction with real-time echocardiography) during stressful exercise. An abnormal ECG and/or “stress echo” is highly predictive of the presence of coronary artery disease and thus the risk of SCA. Similarly, blood chemistry parameters, such as HDL/LDL ratio, homocysteine levels and C-reactive protein and triglyceride levels, when considered together and in the context of age and family history, can also be powerfully predictive of the risk of SCA/D as can be seen in Figure 12, above.
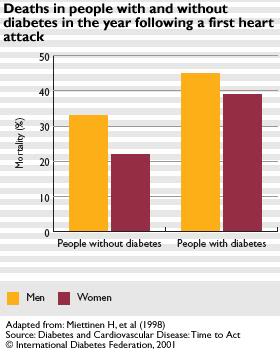
Figure 13: The risk of sudden cardiac death for diabetics in the first year following a myocardial infarction. The risk for male diabetics approaches 50%!
Effort on the part of cryonicists with programming and statistics backgrounds offers the prospect of taking SCA risk factor data from multiple sources and integrating them into a more comprehensive risk factor assessment program. This would allow those cryonicists with the highest risk of SCA to determine if the use of a WCD is appropriate for them.
The cost of the Life-Vest has been impossible for the author to determine so far. Since the device is sold only by prescription (and it is costly), Zoll refuses to discuss a specific “direct consumer retail price,” and instead asks for specific insurance coverage information, or a prescription by a physician. Monthly cost data are available for the UK via the NHS, and the price quoted there is in the range of 27 to 32 pounds sterling per patient, per month.
Interface Capability?
The Life Vest is a less ‘electronically compact’ system and it uses an auditory alarm that is accessible. This raises the possibility that the device can be “hacked” and the alarm output signal be coupled to a mobile phone triggered to dial a pager, or another number where 24/7/365 emergency notification service. It may even be possible to work with Zoll to custom engineer such a device as a plug-in to the unit, as long as it was not being added for any medical indication. Cryonics should still qualify as being ‘exempt’ from being a medical indication, since notification that a person is dead or dying, with no intent to render medical services, is not currently considered medicine. Obviously, if the device delivers a shock and the cryonicist is still alive, the cryonics organization will disregard the alert from the device.
Summary
A variety of FDA approved medically approved devices to detect and treat SCA are now on the market in both the US and Europe. Rapid technological advance in microelectronics and telecommunications has made these devices small enough and lightweight enough to be considered for use by cryonicists wishing to avoid the predicament of experiencing SCA, with or without a long delay to discovery. Careful consideration to adopting the use of a WCD should be given by cryonicists who are 75 or older, or who have specific medical conditions, or other risk factors that put them at high risk of SCA. Intangible factors, such as living alone, which greatly amplifies the risk of remaining undiscovered for prolonged periods of time following SCA, and the increased sense of personal security that may result from use a WCD[18] should also be factored into the decision to use or forego a WCD, or an SCA detection device.
References
1. Harper R, Donnelly N, McCullough I, Francey J, Anderson J, McLaughlin JA, Catherwood PA: Evaluation of a CE approved ambulatory patient monitoring device in a general medical ward. Conf Proc IEEE Eng Med Biol Soc, 2010:94-97.
2. Grace P: Ischaemia-reperfusion injury. Br J Surg 1994 81(5):637-647.
3. Homer-Vanniasinkam S, Crinnion JN, Gough MJ: Post-ischaemic organ dysfunction: a review. Eur J Vasc Endovasc Surg 1997, 14(3):195-203.
4. Mullie A, Van Hoeyweghen R, Quets A: Influence of time intervals on outcome of CPR. The Cerebral Resuscitation Study Group. Resuscitation 1989, 17 Suppl:S23-33; discussion S199-206.
5. Becker LB, Ostrander MP, Barrett J, Kondos GT: Outcome of CPR in a large metropolitan area–where are the survivors? Ann Emerg Med 1991, 20(4):355-361.
6. Martens PR, Mullie A, Calle P, Van Hoeyweghen R: Influence on outcome after cardiac arrest of time elapsed between call for help and start of bystander basic CPR. The Belgian Cerebral Resuscitation Study Group. Resuscitation 1993, 25(3):227-234.
7. Swor RA, Boji B, Cynar M, Sadler E, Basse E, Dalbec DL, Grubb W, Jacobson R, Jackson RE, Maher A: Bystander vs EMS first-responder CPR: initial rhythm and outcome in witnessed nonmonitored out-of-hospital cardiac arrest. Acad Emerg Med 1995, 2(6):494-498.
8. Troiano P, Masaryk J, Stueven HA, Olson D, Barthell E, Waite EM: The effect of bystander CPR on neurologic outcome in survivors of prehospital cardiac arrests. Resuscitation 1989, 17(1):91-98.
9. Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM: Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol, 57(7):794-801.
10. Bardy GH, Lee KL, Mark DB, Poole JE, Toff WD, Tonkin AM, Smith W, Dorian P, Yallop JJ, Packer DL et al: Rationale and design of the Home Automatic External Defibrillator Trial (HAT). Am Heart J 2008, 155(3):445-454.
11. Lofgren B, Molgaard O, Pedersen AK, Krarup NH: [Resuscitation with automatic external defibrillator]. Ugeskr Laeger 2007, 169(42):3584-3585.
12. Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R et al: Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation, 122(12):1144-1152.
13. Celiker A, Olgun H, Karagoz T, Ozer S, Ozkutlu S, Alehan D: Midterm experience with implantable cardioverter-defibrillators in children and young adults. Europace, 12(12):1732-1738.
14. Hauser RG, Maisel WH, Friedman PA, Kallinen LM, Mugglin AS, Kumar K, Hodge DO, Morrison TB, Hayes DL: Longevity of Sprint Fidelis implantable cardioverter-defibrillator leads and risk factors for failure: implications for patient management. Circulation, 123(4):358-363.
15. Adabag AS, Luepker RV, Roger VL, Gersh BJ: Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol, 7(4):216-225.
16. Dai SM, Zhang S, Chen KP, Hua W, Wang FZ, Chen X: Prognostic factors affecting the all-cause death and sudden cardiac death rates of post myocardial infarction patients with low left ventricular ejection fraction. Chin Med J (Engl) 2009, 122(7):802-806.
17. Schott R: Wearable defibrillator. J Cardiovasc Nurs 2002, 16(3):44-52.
18. Beauregard LA: Personal security: Clinical applications of the wearable defibrillator. Pacing Clin Electrophysiol 2004, 27(1):1-3.
19. Reek S, Meltendorf U, Klein HU: [A wearable defibrillator for patients with an intermittent risk of arrhythmia]. Dtsch Med Wochenschr 2002, 127(41):2127-2130.
20. Morrison D, Smith J: Taking a vested interest in a wearable cardioverter defibrillator. Nursing 2009, 39(6):30-32.
21. Auricchio A, Klein H, Geller CJ, Reek S, Heilman MS, Szymkiewicz SJ: Clinical efficacy of the wearable cardioverter-defibrillator in acutely terminating episodes of ventricular fibrillation. Am J Cardiol 1998, 81(10):1253-1256.
22. Chung MK, Szymkiewicz SJ, Shao M, Zishiri E, Niebauer MJ, Lindsay BD, Tchou PJ: Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol, 56(3):194-203.
23. Feldman AM, Klein H, Tchou P, Murali S, Hall WJ, Mancini D, Boehmer J, Harvey M, Heilman MS, Szymkiewicz SJ et al: Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol 2004, 27(1):4-9.
24. Straus S, Bleumink, GS, Dieleman JP, van der Lei, J, Stricker, BH, Sturkenboom, MC.: The incidence of sudden cardiac death in the general population. J Clin Epidemiol 2004 57(1):98-102.
25. Calleja AM, Dommaraju S, Gaddam R, Cha S, Khandheria BK, Chaliki HP: Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol, 105(8):1159-1163.
26. Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD: Diabetes and the risk of sudden cardiac death, the Atherosclerosis Risk in Communities study. Acta Diabetol, 47(Suppl 1):161-168.
Selected Bibliography: Wearable Cardiac Defibrillators
1. Auricchio et al., “Clinical Efficacy of the Wearable Cardioverter-Defibrillator in Acutely Terminating Episodes of Ventricular Fibrillation,” Am J Card, 1998, 81(10):1253-1256.
2. Meltendorf et al., “The Wearable Defibrillator ?a new Method to prevent Sudden Death,” PACE, 2000; 23(4, part II):606.
3. Reek et al., “The Wearable Cardioverter Defibrillator (WCD®) for the prevention of sudden cardiac death ?a single center experience,” Z Kardiol, 2002; 91:1044?052.
4. Schott, “The Wearable Defibrillator,” J Cardiovasc Nurs, 2002; 16(3):44-52.
5. Reek et al., “Clinical Efficacy of the Wearable Defibrillator in Acutely Terminating Episodes of Ventricular Fibrillation Using Biphasic Shocks,?PACE, 2002, 25(4, part II):577.
6. Reek et al. “A wearable defibrillator for patients with an intermittent risk of arrhythmia,” Dtsch Med Wochenschr, 2002; 127(41):2127-30.
7. Capucci et al. “Cost-effective use of a new wearable cardioverter defibrillator to protect patients at risk of SCA,” Europace, 2003, 4(Supplement 1):A44-A45.
8. Gasparini et al. “A new wearable defibrillator: Initial single center experience,” Europace, 2003, 4(Supplement 1):A45.
9. Pignatelli et al. “Use of a new wearable cardioverter defibrillator to reduce the risk of sudden cardiac death,” Europace, 2003, 4(Supplement 1):A45-A46.
10. Suzzani P et al. “The lifecor lifevest™ wearable cardioverter defibrillator,” Europace, 2003, 4(Supplement 1):A46.
11. Lang et al., “Morbidity and Mortality of UNOS Status 1B Cardiac Transplant Candidates at Home,” J Heart Lung Transplant, 2003; 22:419-426.
12. Reek et al., “Clinical Efficacy of a Wearable Defibrillator in Acutely Terminating Episodes of Ventricular Fibrillation Using Biphasic Shocks,” PACE, 2003, 26:2016-2022.
13. Beauregard, “Personal Security: Clinical Applications of the Wearable Defibrillator,” PACE, 2004; 27(1):2-3.
14. Feldman et al., “Use of a Wearable Defibrillator in Terminating Tachyarrhythmias in Patients at High Risk for Sudden Death: Results of WEARIT/BIROAD,” PACE, 2004, 27:4-9.
15. Chung et al., “Robust Long-Term Cardiac Monitoring Using Dry, Non-adhesive Capacitive Electrodes,” JACC, 2004; 43(5, suppl A):141A.
16. Joseph et al., “Compliance and Effectiveness of the Wearable Defibrillator Vest,” JACC, 2004; 43(5, suppl A):300A.
17. Meltendorf, “Using the Wearable Cardioverter Defibrillator a strategy for bridging high risk patients after CABG,” Heart Rhythm, 2005; 2(5, suppl):S32.
18. Wase, “Wearable Defibrillators: A New Tool in the Management of Ventricular Tachycardia/Ventricular Fibrillation,” EP Lab Digest, 2005; 12:22-24.
19. Szymkiewicz et al., “Analysis of Sudden Cardiac Arrests During Wearable Defibrillator Use,” Circulation, 2006; 114 (18, suppl II):II-349.
20. Gronda et al., “Heart Rhythm Considerations in Heart Transplant Candidates and Considerations for Ventricular Assist Devices: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates?006,” J Heart Lung Transplant, 2006; 25(9):1043-1056.
21. Losordo et al., “Intramyocardial Transplantation of Autologous CD34+ Stem Cells for Intractable Angina: A Phase I/IIa Double-Blind, Randomized Controlled Trial,” Circulation, 2007; 115:3165 – 3172.
22. Traub et al., “Sudden cardiac arrest aborted by a wearable cardioverter-defibrillator in newly diagnosed non-ischemic cardiomyopathy,” Heart Rhythm, 2007; 4(5, suppl):S101.
23. Choudhuri et al., “Arrhythmic Events During the 40/90 days ‘Cooling Off’ Period: Clinical Utility of the Wearable Defibrillator,” Circulation, 2007; 116: II_348.
24. Wang et al., “Ventricular Fibrillation remains the Primary Presenting Rhythm: Results from the Wearable Cardioverter Defibrillator Human Study,” Circulation, 2007; 116: II_934 – II_935.
25. Freeman et al., “Is Severe Post-shock Bradyarrhythmia In Patients Using Wearable Defibrillators Common or Serious?” Circulation, 2007; 116: II_931.
26. LaPage et al., “A Fatal Device-Device Interaction between a Wearable Automated De?brillator and a Unipolar Ventricular Pacemaker,” PACE, 2008; 31:912?15.
27. Abi-Samra et al., “Wearable Defibrillator: Effective Bridging Substitute for ICD Implants,” Europace, 2008; 10: i160.
28. Mortada et al., “Sudden Cardiac Death” in: Jeremias A, Brown DL (eds). Cardiac Intensive Care, 2nd Edition, Elsevier, St. Louis (in press).
29. Szymkiewicz, et al. “A Comparison of Compliance and Effectiveness of Wearable Defibrillators and Home AEDs in Out-of-Hospital Sudden Cardiac Arrest,” Circulation, 2008; 118: S_1466.
30. Lewicke et al., “Exploring QT interval changes as a precursor to the onset of ventricular fibrillation/tachycardia,” Journal of Electrocardiology, 2009; 42(4):374-9.
31. Szymkiewicz, et al., “Incidence and causes of inappropriate defibrillation during wearable defibrillator use,” Heart Rhythm, 2009; 6(5): S74.
32. Morrison et al., “Taking a vested interest in a wearable cardioverter defibrillator,” Nursing, 2009; 39(6):30-2.
33. Dillon et al., “An evaluation of the effectiveness of a wearable cardioverter defibrillator detection algorithm,” Journal of Electrocardiology, 2009; (June, electronic ahead of print).
34. Saltzberg et al., “Characteristics of Peripartum Cardiomyopathy Patients Using a Wearable Cardiac Defibrillator,” Journal of Cardiac Failure, 2009; 15(6):S59.
35. Lee et al., “Role of wearable and automatic external defibrillators in improving survival in patients at risk for sudden cardiac death,” Curr Treat Options Cardiovasc Med, 2009;11(5):360-5.
36. Klein et al., “Bridging a Temporary High Risk of Sudden Arrhythmic Death. Experience with the Wearable Cardioverter Defibrillator (WCD),” PACE, 2009 Nov 2 [Epub ahead of print]
37. Prochnau, “Successful use of a wearable cardioverter-defibrillator in myocarditis with normal ejection fraction,” Clin Res Cardiol, 2009 Nov 17. [Epub ahead of print]
38. Zareba et al., “Sudden Cardiac Arrest in End-Stage Renal Disease: Successful Resuscitation With Wearable Cardiac Defibrillator,” Circulation, Nov 2009; 120: S701 – S702.
39. Klein et al. “The Wearable Cardioverter Defibrillator: Bridge to the Implantable Defibrillator,” Cardiac Electrophysiology Clinics, 2009;1(1):129-46.
40. Everitt et al., “Use of the Wearable External Cardiac Defibrillator in Children,” PACE, 2010 Feb 23. [Epub ahead of print].
41. Chung et al. “Aggregate national experience with the wearable cardioverter-defibrillator vest: event rates, compliance and survival,” JACC, 2010;55(10 suppl 1):A10.
[1] Polar is the leading manufacturer of heart rate monitoring sports watches and the bundled Polar-Nokia product is featured here: http://www.intomobile.com/2009/01/14/nokia-n79-active-bundles-polar-heart-monitor-for-health-fanatics/
[2] OEM stands for original equipment manufacturer, but in reality means a manufacturer of equipment that may be marketed by another manufacturer!
[3] Which the author most emphatically does not recommend for use in cryonics!
